A new paper published in the open-access journal Polymers has investigated the use of chitosan gel polymer electrolytes in lithium-ion batteries. The research has been conducted by scientists from Hainan University and Foshan University in China.

Image Credit: Smile Fight/Shutterstock.com
Lithium-ion Batteries: Advantages and Drawbacks
Lithium-ion batteries, along with fuel cells and supercapacitors, are one of the key technologies driving the electrification of society and industry and overcoming the environmental damage of fossil fuel use.
Aside from their environmental friendliness compared to fossil fuels, lithium-ion batteries possess the key benefits of long cycle lives and high energy density, making them ideal high-performance electrochemical energy storage technologies. However, there are some key drawbacks to these devices which are linked to currently employed electrolytes.
Lithium-ion batteries can suffer from critical safety and technical issues, such as the risk of explosion, lithium dendrite growth, short-circuit risk, electrolyte leakage, and thermal runaway. To overcome these issues, research has focused on the development of novel electrolytes.
Polymer Electrolytes
In recent years, polymer electrolytes have emerged as candidate materials for lithium-ion batteries. Solid polymer electrolytes have piqued the interest of scientists working in the field of battery research due to their non-flammability and solid state. However, they still suffer from key drawbacks, including their low ionic conductivity and high interfacial resistance.
Due to solid polymer electrolytes possessing these limitations, which hinder their full commercialization, research has turned instead to gel polymer electrolytes. These innovative electrolytes combine the advantages of solid polymer electrolytes and conventional liquid electrolytes and provide a route toward the rational design of lithium-ion batteries with improved safety and performance.
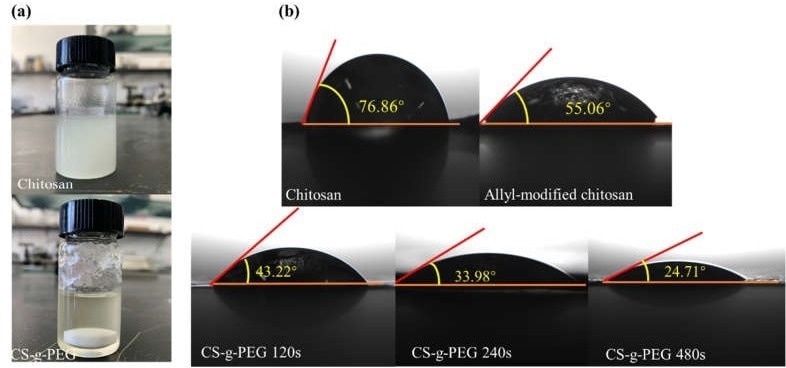
(a) Photographs of chitosan and CS-g-PEG dissolved in DMSO. (b) Contact angle measurements between liquid electrolyte and chitosan, allyl-chitosan and CS-g-PEG 120, 240 and 480 films. Image CredI=it: Wang, A et al., Polymers
High-molecular-weight polymers in gel polymer electrolytes form a skeletal structure, which optimizes their mechanical strength, electrochemical stability, and thermal stability. Moreover, they possess excellent ionic conductivity due to the plasticizing effect of solid polymers by liquid electrolytes.
Several polymers have been investigated for the construction of gel polymer electrolytes, including PAN, PMMA, and PEO. PEO-based gel polymer electrolytes have emerged as forerunners in research. Whilst the development of synthetic polymer-based gel electrolytes has proven promising, there are key environmental issues with their manufacture that limit their sustainability.
Organic Polysaccharide-derived Polymer Electrolytes
To overcome these environmental drawbacks and improve the green credentials of gel polymer electrolyte research, scientists have turned to the exploitation of naturally occurring organic polymers. Polymeric materials constructed from polysaccharides, including cellulose, starch, and chitin, have been the focus of study in this field of battery research.
Organically derived polymers have several advantages, such as biocompatibility, non-toxicity, biodegradability, and superior chemical resistance. Moreover, chitosan, a linear polysaccharide derived from chitin, can be formed into multiple functional architectures such as hydrogels, nanofibers, and porous scaffolds.
Some studies have reported the use of chitosan for gel polymer electrolytes in aqueous supercapacitors. Their use in alkali metal batteries has been limited by poor dissolubility of chitosan and reduced affinity of chitosan to liquid electrolytes. Some researchers have blended chitosan with other polymers, such as PEO, but these studies have produced solid electrolytes. Currently, studies on modifying chitosan gel polymer electrolytes are limited.
The Study
The new paper in Polymers has produced a chitosan-incorporated gel polymer electrolyte with high ionic conductivity and a high lithium-ion transport number at room temperature. A simple UV-induced reaction method was used to prepare a PEGylated chitosan, which was designed using chemical grafting methods.
UV irradiation time was employed as a control variable in the research. By controlling this variable, the authors could synthesize electrolytes with different degrees of PEG grafting. The novel PEGylated chitosan possesses an enhanced affinity with liquid electrolytes, which overcomes previously reported limitations in research.
Rapid lithium-ion transport is facilitated in the electrolyte by the complexation of ether bonds with lithium ions, and the proposed PEGylated chitosan can satisfy the ion transport requirements of lithium-ion batteries. A button cell was constructed to evaluate the performance of the electrolyte, displaying favorable results.
Interfacial compatibility is improved by increasing the PEG content due to decreased interfacial resistance. A stable interface between electrolyte and lithium electrolytes was confirmed over a 14-day testing period, with a relatively stable state reached within seven days.
The authors also reported a satisfactory initial discharge capacity. Moreover, batteries constructed with this novel gel polymer electrolyte can achieve a long cycle performance, which warrants further exploration.
Overall, this research has demonstrated the rational design of a gel polymer electrolyte incorporating chitosan, an organic-based, sustainable, and environmentally friendly polymer. The satisfactory electrochemical performance and safety improvements observed in the material could provide opportunities for the design of high-performance, safe, and environmentally sustainable lithium-ion batteries.
More from AZoM: How are Graphene Batteries Made?
Further Reading
Wang, A et al. (2022) A PEGylated Chitosan as Gel Polymer Electrolyte for Lithium Ion Batteries Polymers 14(21) 4552 [online] mdpi.com. Available at: https://www.mdpi.com/2073-4360/14/21/4552
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.