 By Stefano Tommasone Ph.D.Reviewed by Lexie CornerJan 7 2025
By Stefano Tommasone Ph.D.Reviewed by Lexie CornerJan 7 2025Unlike traditional batteries, which rely on the movement of metal ions such as lithium, proton batteries use hydrogen ions to store and transfer energy. This innovation addresses key challenges posed by existing battery technologies, including sustainability, resource scarcity, and environmental impact.
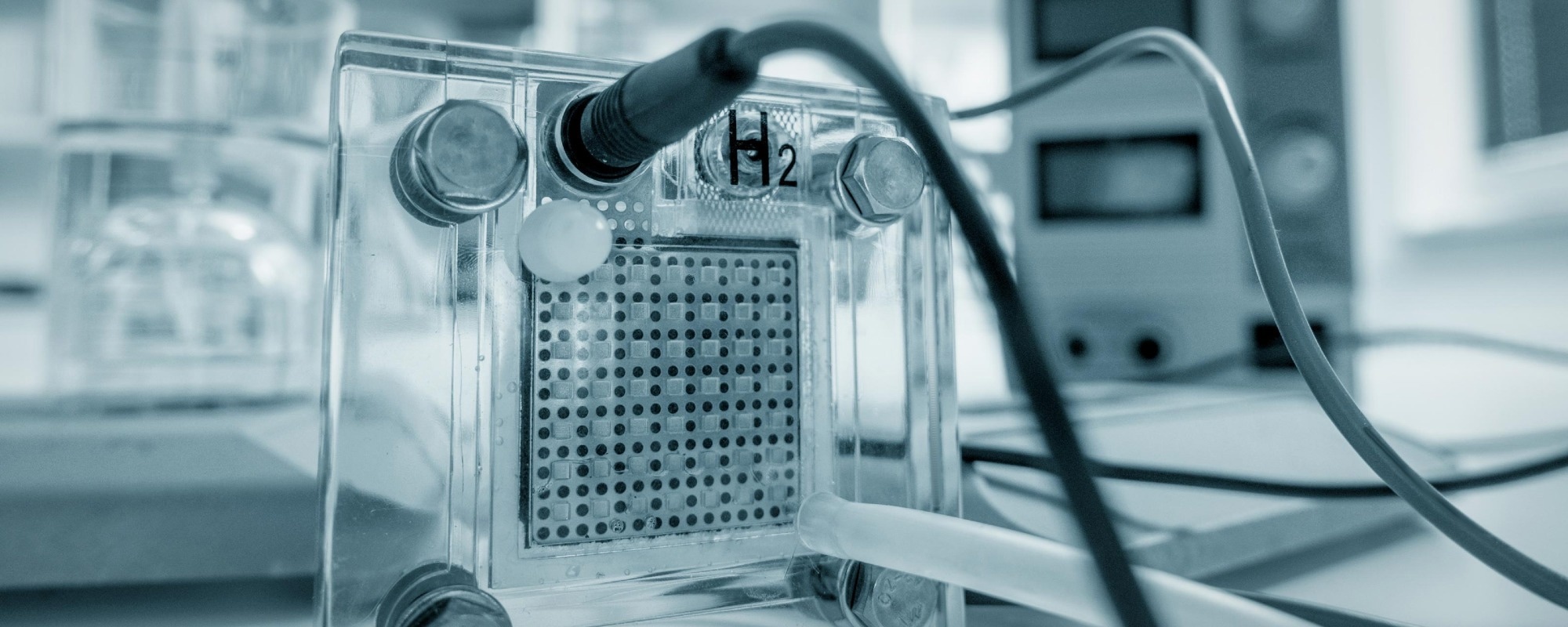
Image Credit: luchschenF/Shutterstock.com
There is a growing need for alternative battery technologies. While lithium-ion batteries are widely used, they depend on resources with limited availability, such as lithium and cobalt, whose extraction raises environmental and ethical concerns. Additionally, lithium-ion batteries face limitations in energy density, safety, and long-term performance.
Proton batteries, by contrast, utilize abundant materials and produce water as their primary byproduct, offering a potentially greener, safer, and more efficient energy storage solution. These attributes make them particularly appealing for applications such as grid-scale energy storage and electric vehicles.1
Working Principle
Proton batteries transfer protons through a selective membrane, where they combine with electrons and oxygen to generate energy. The process relies on three key components: the anode, the proton-exchange membrane, and the cathode.
- Anode: The anode typically consists of hydrogen-rich compounds or organic materials capable of releasing protons. One example is tetraamino-benzoquinone (TABQ), which is lightweight and efficient in storing and releasing hydrogen ions.
- Membrane: The proton-exchange membrane selectively allows protons to pass through while blocking other ions. Nafion, a sulfonated tetrafluoroethylene-based fluoropolymer, is commonly used due to its high proton conductivity and chemical stability.
- Cathode: At the cathode, protons recombine with electrons from the external circuit and oxygen from the air, forming water as a byproduct. This reaction releases energy and completes the electrochemical cycle.
The process begins with the anode releasing protons, which are transported through the membrane. While electrons flow through an external circuit to generate electricity, the protons pass through the membrane to the cathode. There, they recombine with electrons and oxygen, creating water and releasing energy. This precise mechanism enables efficient energy conversion.
Proton batteries can be categorized into two types:
Proton rocking-chair batteries: Both electrodes rely on processes like proton intercalation reactions, surface reactions, and ion adsorption.
Hybrid proton batteries: These involve hybrid processes where one electrode stores protons, while the other uses metal-based conversion reactions, such as those involving manganese or lead.2
Advantages and Disadvantages of Proton Batteries
Proton batteries provide several significant advantages over traditional metal-ion battery technologies. They use resources that are more readily available and less harmful to the environment. Hydrogen, a key component of proton batteries, is significantly more abundant in the Earth’s crust than lithium or cobalt. These batteries also produce water as a primary byproduct, offering a more sustainable energy storage solution.
The small size and low atomic mass of protons enable a high density of charge carriers within the electrolyte, enhancing overall battery performance. This is further supported by the Grotthuss mechanism, which facilitates proton transfer via hydrogen bonding, leading to improved ionic conductivity and energy efficiency.
These features enable proton batteries to achieve stable cycling, high energy density, and faster recharging compared to lithium-ion batteries. Moreover, the organic materials used in proton batteries degrade less than metal-based electrodes, resulting in a longer lifespan with more charge-discharge cycles.
Proton batteries also perform well in low-temperature environments, unlike many metal-ion batteries. This advantage is attributed to the low freezing point of acidic electrolytes, making proton batteries suitable for a wider range of operating conditions.
Despite their many advantages, proton batteries remain in the experimental phase and face several significant challenges. One major hurdle is cost. Advanced materials such as Nafion, which are currently used in proton batteries, increase production expenses. Identifying cost-effective alternatives is essential for large-scale adoption.
Cycling stability is another area of concern. Poor stability, often caused by interactions between the solvent and electrode surfaces, can limit the lifespan of these batteries. Designing improved electrolytes to suppress these interactions and protect electrode structures is critical for enhancing durability.
More from AZoM: New Horizons in Battery Quality and Cathode Materials
Recent Developments
Recent advances have highlighted the potential of proton batteries. A prototype based on an organic electrode material demonstrated long-term stability, achieving over 3500 charge-discharge cycles with minimal degradation. This showcases significant progress in both energy density and lifespan.3
Efforts are also underway to develop cost-effective and durable alternatives to Nafion. Bio-based polymers and synthetic compounds, such as inorganic perovskite oxides and solid oxide materials like HSrCoO2.5, offer promising pathways for reducing costs without sacrificing performance.4
Research into new electrolytes and electrode materials could improve battery performance and energy storage capacity.5 Studies have shown that a "water-in-sugar" electrolyte, created by dissolving glucose in highly concentrated acids, can stabilize the electrode structure and extend the working potential.6
In the future, artificial intelligence and machine learning may accelerate the discovery of new electrode materials and electrolytes. These technologies could optimize molecular designs and identify cost-effective solutions, although the current dataset for proton batteries remains limited.
References and Further Reading
- Su, Z., Guo, H., Zhao, C. (2023). Rational Design of Electrode-Electrolyte Interphase and Electrolytes for Rechargeable Proton Batteries. Nanomicro Lett. 10.1007/s40820-023-01071-z. https://link.springer.com/article/10.1007/s40820-023-01071-z
- Wu, S., Guo, H., Zhao, C. (2024). Challenges and Opportunities for Proton Batteries: From Electrodes, Electrolytes to Full-Cell Applications. Advanced Functional Materials.https://doi.org/10.1002/adfm.202405401. https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.202405401
- Wu, S., Taylor, M., Guo, H., Wang, S., Han, C., Vongsvivut, J., Meyer, Q., Sun, Q., Ho, J., Zhao, C. (2024). A High-capacity Benzoquinone Derivative Anode for All-organic Long-cycle Aqueous Proton Batteries. Angewandte Chemie International Edition. https://doi.org/10.1002/anie.202412455. https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202412455
- Lu, N., Zhang, Z., Wang, Y., Li, H.-B., Qiao, S., Zhao, B., He, Q., Lu, S., Li, C., Wu, Y., Zhu, M., Lyu, X., Chen, X., Li, Z., Wang, M., Zhang, J., Tsang, S. C., Guo, J., Yang, S., Zhang, J., Deng, K., Zhang, D., Ma, J., Ren, J., Wu, Y., Zhu, J., Zhou, S., Tokura, Y., Nan, C.-W., Wu, J. Yu, P. (2022). Enhanced low-temperature proton conductivity in hydrogen-intercalated brownmillerite oxide. Nature Energy.10.1038/s41560-022-01166-8 https://doi.org/10.1038/s41560-022-01166-8
- Wu, S., Guo, H., Su, Z., Jia, C., Zhang, X., Wang, S., Zhao, T., Meyer, Q. Zhao, C. (2024). Suppressed Manganese Oxides Shuttling in Acidic Electrolytes Extends Shelf-Life of Electrolytic Proton Batteries. Advanced Functional Materials.https://doi.org/10.1002/adfm.202315706 https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.202315706
- Su, Z., Chen, J., Ren, W., Guo, H., Jia, C., Yin, S., Ho, J. Zhao, C. (2021). “Water-in-Sugar” Electrolytes Enable Ultrafast and Stable Electrochemical Naked Proton Storage. Small. https://doi.org/10.1002/smll.202102375 https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.202102375
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.