Increased backlogs of seized drug samples, rapid turnaround times, and the emergence of many novel psychoactive substances (NPS) are major challenges for the forensic community worldwide.
This article demonstrates a comprehensive workflow provided by the Seized Drug Suite, which incorporates the powerful combination of Direct Analysis in Real Time and High Resolution Mass Spectrometry (DART-HRMS).
This unparalleled pairing combines ultra-fast acquisition speed with high information depth for targeted and non-target analyses.
Introduction
An ever-increasing number of seized drug samples, as well as a constant influx of novel psychoactive substances, pose challenges to forensic labs around the world.
The NFLIS-Drug 2019 Survey of Crime Laboratory Drug Chemistry Sections Report revealed that laboratories have an average turnaround time of 60 days and a backlog of 1,800 drug cases.1 This demonstrates that established conventional techniques are being pushed to their limits by the current demand for sample throughput.
A potential alternative is to use Direct Analysis in Real Time in combination with High Resolution Mass Spectrometry (DART-HRMS) for screening analysis. Early adopters have already praised DART-MS for its ability to produce reliable analytical results much faster and more easily than traditional techniques.2
What has been lacking so far is the seamless integration of DART into routine testing workflows.
This article demonstrates the use of DART-HRMS to investigate paper samples seized during routine controls in prisons and forensic psychiatry institutions. A common method of smuggling drugs into prison involves soaking letter-writing paper in a drug mixture, allowing it to dry, and then writing on it.
The prisoner receives the letter and reads it, then smokes portions of it to consume the drug. To counteract this approach, authorities have developed methods for screening letters sent into prison.
Fast Seized Drug Screening with the Seized Drug Suite
Analysis of Authentic Paper Samples
The Seized Drug Suite was used to screen paper samples seized during routine controls in prisons and forensic psychiatric institutions. The Seized Drug Suite is a hardware and software package that includes optimized methods for acquisition, processing, and reporting designed specifically for the analysis of seized drug samples.
The samples were extracted with MeOH and 3 μL of the extract was placed on a DART QuickStrip® card. All analyses were performed using a DART JumpShot® source and a compact QTOF mass spectrometer (both from Bruker).
Helium was used as an ionization gas at 275 degrees Celsius. Data was collected in positive ionization mode with AutoMS/MS at 24 eV and 36 eV collision energies.
TargetScreener HR was used for subsequent LC-MS confirmation analysis after an Elute UHPLC was connected to the compact QTOF instrument. The simple exchange from the DART source to the Vacuum Insulated Probe-Heated ElectroSpray Ionization (VIP-HESI) source takes only a few minutes and does not break the vacuum.
Data Acquisition
The Bruker compact QTOF instrument's fast data acquisition speed enables it to receive full scan MS information as well as MS/MS fragmentation spectra within the DART analysis's very short run time, which is typically 3 s to 60 s.
During the acquisition, the compact QTOF mass spectrometer switches between MS and data-dependent AutoMS/MS at high collision energy. AutoMS/MS parameters were carefully tuned to obtain high-quality fragment data on the most selected precursors.
The "active exclusion" parameter ensures fragmentation of low-abundant precursor ions in the presence of hi-abundant precursors enabling maximum coverage of precursor ions selected for MS/MS.
Spectral Library Search
Spectral library searches help identify compounds. An open library concept allows libraries to be chosen freely and integrated into the workflow. The Seized Drug Suite includes a spectral library containing MS/MS spectra for over 280 compounds obtained using DART-QTOF-MS.
Because there is no significant difference in fragmentation patterns between DART and ESI, there is no reason to use only DART-specific libraries. In addition, custom spectral libraries can be built and modified.
The NIST DART-MS Forensic Database (2021), the "Maurer, Meyer, Helfer, Weber: LC-HR-MS/ MS Library of Drugs, Poisons, and Their Metabolites" and the NIST/EPA/NIH Mass Spectral Library 2023 are among the libraries available.
Sample Report
The sample report is generated automatically and contains all relevant information, such as a data table with sample and method information, an overview of the library search results, and spectral details for each compound found.

Image Credit: Bruker Applied Mass Spectrometry
Overview Of the Sample Preparation Process, Hardware and Software Used
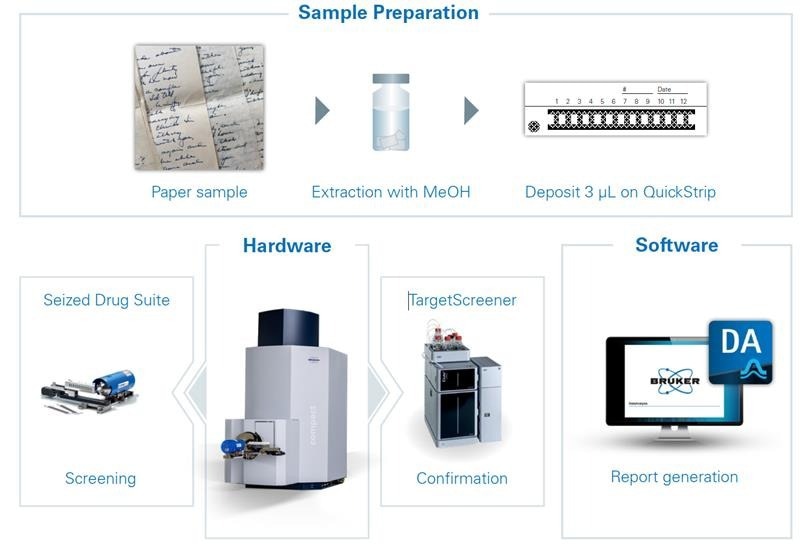
Figure 1. Overview of the sample preparation process, hardware and software applied. Image Credit: Bruker Applied Mass Spectrometry
Workflow for Screening of Seized Drug Samples with DART-HRMS/MS
Full scan MS and data-dependent MS/MS information are acquired in parallel. A sample analysis time of 12 seconds results in up to 120 MS/MS spectra.
TargetScreener is used for confirmation of the results (see Figure 2). It provides a comprehensive screening solution, including a high-quality, regularly updated forensic database of over 1,000 compounds with forensic relevance.
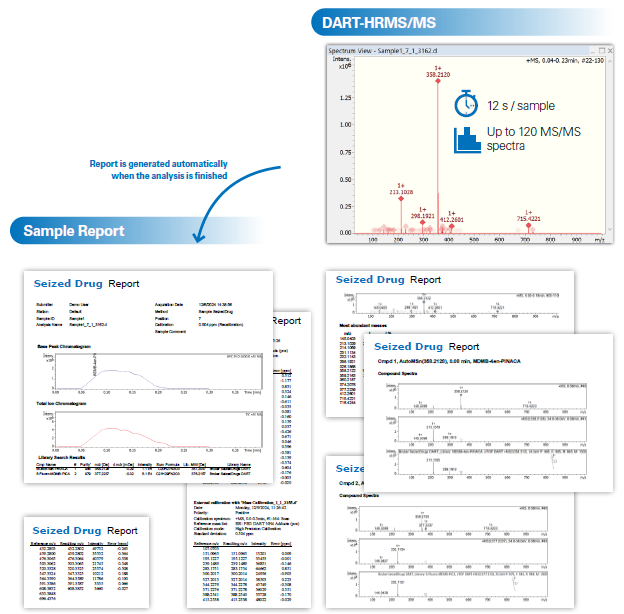
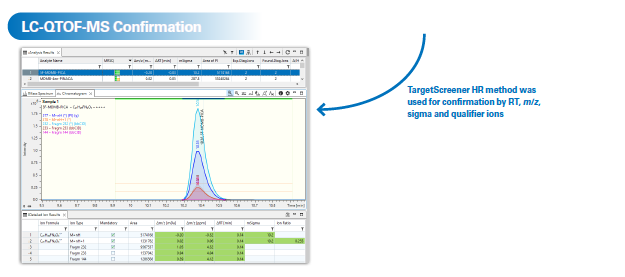
Figure 2. Workflow for screening of seized drug samples using DART-HRMS. Image Credit: Bruker Applied Mass Spectrometry
Results
Authentic paper samples were analyzed using the Seized Drug Suite workflow. Figure 2 shows the results for one selected paper sample. Data acquisition was completed in 12 seconds, yielding approximately 120 individual MS/MS spectra.
Two synthetic cannabinoids, MDMB-4-en-PINACA and 5-Fluoro-MDMB-PICA, were successfully identified using library match scores greater than 900. Both were later confirmed through LC-QTOF-MS analysis. Synthetic cannabinoids were identified as the dominant drug effect group across all analyzed paper samples.
This group includes substances that function similarly to THC (d9-tetrahydrocannabinol) in that they interact with cannabinoid receptors. In recent years, synthetic cannabinoids have taken the lead among NPS. From December 2021 to May 2023, 40% of newly reported NPS were synthetic cannabinoids.3
As of 2022, MDMB-4-en-PINACA was the most frequently reported synthetic cannabinoid in the United States, with 5-Fluoro-MDMB-PICA ranking third.4
Identification of Unknowns
With the constant emergence of these NPS, it is common for seized drug samples to produce signals that do not match any existing library entries but may still be forensically relevant. Another significant advantage of high-resolution mass spectrometers is their ability to identify unknown compounds.
QTOF mass spectrometers measure the mass of an ion with greater accuracy than low-resolution instruments such as quadrupoles, allowing for the determination of the elemental composition of unknown compounds.
Matching the experimental isotope pattern of the discovered elemental composition to the theoretical one provides a second criterion for validating the formula prediction.
MS/MS fragmentation spectra provide structural information that can be compared to in-silico fragmentation patterns of compound structures found in databases. As a result, compound identification can be narrowed down step by step using these three criteria.
MetaboScape®, Bruker's software, guides the user through a seamless three-step workflow from initial spectral features to final annotations in an automated and efficient manner. Below is a detailed walkthrough of this process, with the annotation of the synthetic cannabinoid ADB-BUTINACA as an example.
First, MetaboScape's SmartFormula tool lists the best-matching elemental compositions based on the calculated accurate mass and isotope patterns. Here, the user can specify which elements should be considered.
In the following step, the CompoundCrawler tool searches publicly available databases like PubChem and a local, customizable database called AnalyteDB for structure candidates that correspond to the elemental compositions.
To address the issue of structures having to be listed in public databases for CompoundCrawler to detect them, AnalyteDB allows users to incorporate their own structures directly at the local level. Such new structures could be derived from deep learning or AI-based predictions.
As a final step, the discovered structure candidates are suspected of in-silico fragmentation by MetFrag5 and compared to the experimental MS/MS spectrum. The workflow described here represents a standard-free annotation approach by utilizing in-silico fragmentation.
This is especially useful for NPS, where reference standards are not always readily available. After successfully identifying a new compound, it can be saved in the spectral library for future comparisons and searches.
Using the principle of 'all data is collected all the time', retrospective evaluation of previously analyzed samples is possible without repeating any experimental work.
Three-Step Workflow for the Unknown Annotation in Metaboscape.
The identification of ADB-BUTINACA without the need for a standard is presented below.
SmartFormula
SmartFormula calculates possible elemental compositions based on isotope pattern fit and m/z measurements.
CompoundCrawler
CompoundCrawler looks for structure candidates among the proposed elemental compositions.
MetFrag
MetFrag fragments structure candidates in-silico and compares the results to the experimental MS/MS spectrum.
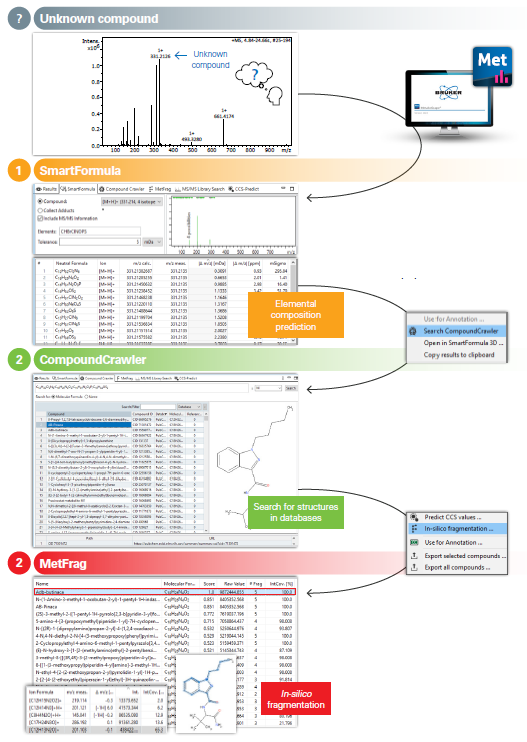
Image Credit: Bruker Applied Mass Spectrometry
Differentiation of Isomeric Synthetic Cannabinoids
Differentiating between isomeric drugs is critical for accurate identification. While chromatography-free methods do not rely on retention time separation, DART-QTOF-MS can effectively distinguish isomeric drugs in various situations by analyzing their fragmentation profiles.
ADB-BUTINACA and AB-PINACA are isomeric synthetic cannabinoids with the same chemical formula C18H26N4O2.
The only structural difference between these compounds is the position of one methyl group. This minor structural difference, however, is sufficient to distinguish them using DART-HRMS/MS based on fragmentation patterns.
The methyl-group in ADB-BUTINACA is located at the part of the molecule that causes the neutral loss, whereas in AB-PINACA, it is located at the detectable fragment ion.
A characteristic fragment ion at m/z 201 and 215 is thus observed, which serves to differentiate the two isomers. The identification of ADB-BUTINACA in the seized paper sample shown in Figure 3 is unambiguous because the fragment ion at m/z 201 was detected.
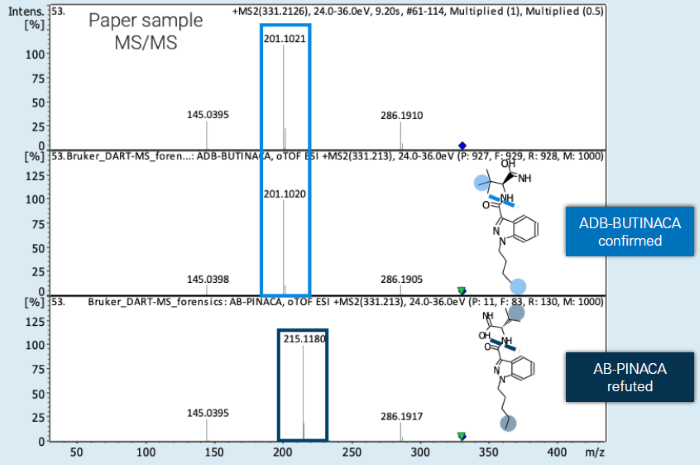
Figure 3. Differentiation of the isomeric synthetic cannabinoids ADB-BUTINACA and AB-PINACA based on their fragmentation pattern. Reference standard-based identification of ADB-BUTINACA in an authentic paper sample. Image Credit: Bruker Applied Mass Spectrometry
Conclusion
DART-HRMS was suitable for a highly accelerated screening of seized drug samples. It offers a viable alternative to traditional techniques that eliminates the need for time-consuming chromatography.
Going chromatography-free with DART saves analysis time per sample to less than a minute and significantly reduces solvent consumption. Bruker's QTOF mass spectrometer combines high detection selectivity with excellent sensitivity.
It can be used for more than just targeted analyses, such as detecting unexpected compounds or identifying unknown compounds. Because it is always in full scan mode, it makes retrospective analysis of any type of investigated sample easier, which is especially useful in the context of NPS.
References
- U.S. Drug Enforcement Administration, Diversion Control Division. (2019). NFLIS-Drug 2019 Survey of Crime Laboratory Drug Chemistry Sections Report. Springfield, VA: U.S. Drug Enforcement Administration
- Sisco, E. and Forbes, T.P. (2021). Forensic applications of DART-MS: A review of recent literature. Forensic Chemistry, 22, p.100294. https://doi.org/10.1016/j.forc.2020.100294.
- United Nations Office on Drugs and Crime, Current NPS Threats, Volume VI, August 2023. Available at: https://www.unodc.org/res/scientists/ewa/Current_NPS_Threats_VI.pdf.
- EMERGING THREAT REPORT Annual 2022 Drug Enforcement Administration Special Testing and Research Laboratory. Available at: https://cesar.umd.edu/sites/cesar.umd.edu/files/pubs/DEA-Emerging-Threat-Report-2022-Annual.pdf (Accessed 13 May 2025).
- Wolf, S., et al. (2010). In silico fragmentation for computer assisted identification of metabolite mass spectra. BMC Bioinformatics, 11(1). https://doi.org/10.1186/1471-2105-11-148.

This information has been sourced, reviewed and adapted from materials provided by Bruker Applied Mass Spectrometry, developed in collaboration with the University of Freiburg.
For more information on this source, please visit Bruker Applied Mass Spectrometry.