Lasers are used in different ways for processing medical devices. Laser marking machines with integrated vision systems emerged as an effective means of identifying medical components, e.g. for marking UDI compliant codes.
Innovative laser technology has become the preferred solution for marking medical devices as it enables medical device manufacturers to overcome marking challenges.
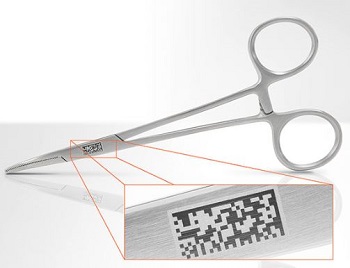
Particularly zero-defect laser marking plays an increasingly important role in manufacturing medical devices since the FDA (Food and Drug Administration) requires that, by 2016, every medical device in the USA contains a 2D code for traceability.
The key to meeting the FDA identification requirements while reducing production costs, minimizing waste and improving product quality is a laser marking system combined with the software concept HELP (Holistic Enhanced Laser Process).
Apart from the marking of the medical product itself, HELP offers pre-mark verification prior to marking and post-mark validation right after marking.
Particularly important for medical device manufacturers: During the unique post-mark verification process, the content of 1D and 2D codes (e.g. Datamatrix [ECC200] GS1 compliant and graded) can be directly read which is indispensable for compliance with the new Unique Device Identifier set by the FDA.
About FOBA
FOBA Laser Marking & Engraving is among the leaders in manufacturing and supplying precision laser systems for marking and engraving. FOBA marking lasers mark a variety of materials and parts not least in the key markets of Automotive and Medical but also in Electronics, Plastics, Safety and ID.
FOBA laser workstations for marking and engraving are especially applied in the fields of Automotive part production and Medical device marking as well as in Tool, Metal and Mold Making, Plastics processing and Jewelry and Coinage. Worldwide sales and service branches service the most important markets.
In September 2009, FOBA has become part of ALLTEC GmbH. Since then, FOBA is part of ALLTEC as a sales channel for laser part marking and engraving.