As a result of lithium-sulfur (Li-S) batteries’ huge energy capacity, they have great potential for use in energy storage systems. But safety issues connected with their thermal behavior continue to be a concern for researchers.
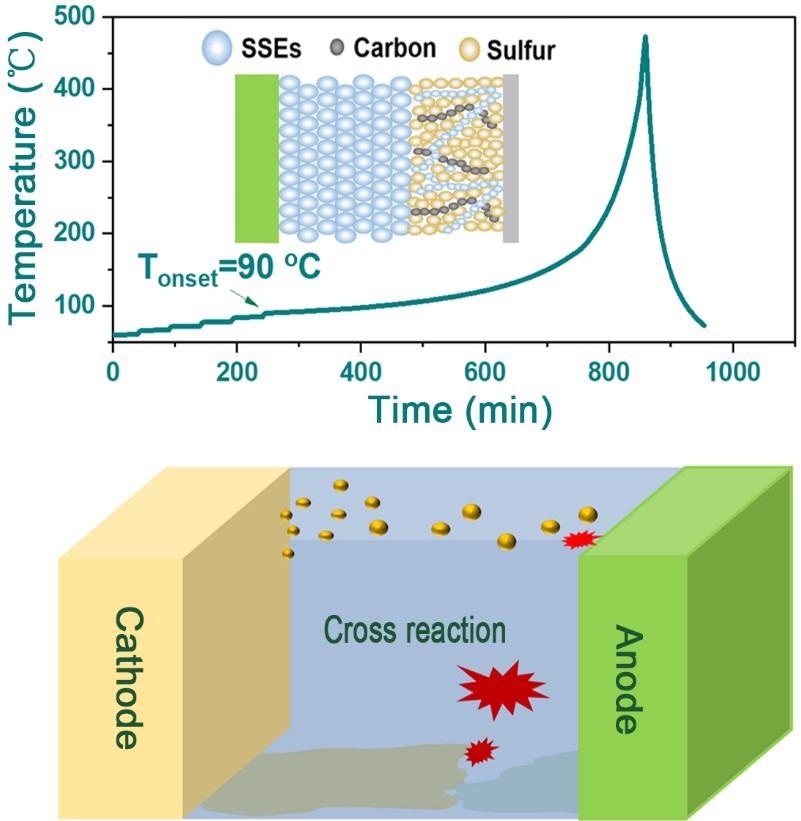 Thermal performance of Li-S cell with thermal stable electrolyte. Image Credit: Lang Huang.
Thermal performance of Li-S cell with thermal stable electrolyte. Image Credit: Lang Huang.
Currently, a research group headed by scientists from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), Chinese Academy of Sciences (CAS) have disclosed that the thermal runaway routes of Li-S batteries might help to fulfill the safety problems of next-generation batteries.
This study was reported on March 14th, 2022, in the journal Joule.
One of these safety concerns with Li-S batteries is thermal runaway, a phenomenon where the battery begins to overheat uncontrollably. As the temperature in the battery rises, the electrolyte can be ignited, possibly leading to a fire.
Lang Huang, Study Lead Author and Assistant Professor, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
The researchers set forth to analyze the safety traits of large-format pouch-cell Li-S batteries. They analyzed inorganic all-solid-state electrolytes due to their high thermal stability. This might offer a strategy for overcoming the safety issues. The thermal runaway behavior of the pouch-cell batteries was also examined.
The outcomes displayed that even all-solid-state electrolytes cannot halt the thermal runaway that takes place in Li-S batteries at high temperatures. This knowledge will benefit researchers who are looking for methods to construct next-generation Li-S batteries that are much safer.
The team analyzed the thermal features of Li-S batteries from the view of the material. They began at the entire pouch cell and worked down to the electrode level.
They found that the Li-S batteries’ exothermic chain reactions were initially activated by sulfur cathode derivatives that reacted with the electrolyte. This reaction was further expedited as the lithium metal anode reacted with the electrolyte or cathode active species.
As the Li metal anode and sulfur cathode melted, they immigrated and cross-react at high temperatures. This plays a decisive role in the thermal runaway behavior of the battery.
The study discloses that as-assembled Li-S batteries with the help of electrolytes of various thermal stabilities, such as inorganic all-solid-state electrolytes, undergo quick thermal runaway due to the inevitable short-circuiting that happens when the Li anode and sulfur cathode melts.
The in-depth depicted thermal runaway routes will shed fresh lights on the way forward for building next-generation Li-S battery with enhanced safety performance.
Guanglei Cui, Professor, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
The study was financially supported by the National Natural Science Foundation of China, Natural Science Foundation of Shandong Province, Priority Research Program of Chinese Academy of Sciences, CAS Key Technology Talent Program, and the Key Scientific, and Technological Innovation Project of Shandong.
Journal Reference:
Huang, L., et al. (2022) Thermal runaway routes of large-format lithium-sulfur pouch cell batteries. Joule. doi.org/10.1016/j.joule.2022.02.015.