PVC possesses several advantageous properties which make it a commonly used material in industries such as construction, clothing, the automotive industry, toymaking, and biomedicine. PVC is lightweight, has low manufacturing costs, is soft, and recyclable. It is usually combined with up to 40% plasticizers to create commercially viable materials with enhanced mechanical properties.
Whilst this material has shown promising applications for medical devices, its widespread medical use is hindered by plasticizers such as DEHP and phthalates. These plasticizers have potential carcinogenic, mutagenic, endocrine-disrupting, and reproductive toxicity issues. Because of this risk, DEHP use in PVC medical devices has been restricted by the EU, with alternative plasticizers recommended.
Plasticizers can interact with both the polymer matrix of medical devices and drug solutions due to their small molecular size. Additionally, leaching and sorption can occur, leading to adverse effects on the patient. Leaching can cause safety issues due to potential toxicity or degradation of the drug’s properties.
Sorption can affect the stability and efficiency of drugs via the loss of active pharmaceutical ingredients, leading to reduced drug responses and difficulty controlling the delivered drug’s concentration. Drug-polymer interactions are a major issue in the delivery of drugs such as insulin, demanding further research in the pharmaceutical industry.
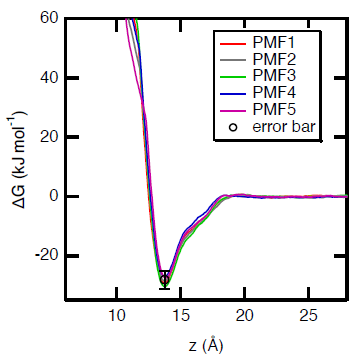
Gibbs free energy profiles calculated during the adsorption of paracetamol onto a polyethylene surface. 5 five independent calculations were performed to estimate the standard statistical fluctuations which are of the order of magnitude of 2-3 kJ mol−1. Image Credit: Sahnoune, M et al., ACS Applied Polymer Materials
Understanding Sorption Behavior
Studies have identified several factors which impact sorption in these materials. The chemical nature and properties of the polymer itself is an important contributory factor, along with the amount and nature of plasticizers used. Furthermore, the drug’s physiochemical properties such as concentration, steric hindrance, and lipophilicity play a role.
Other factors which govern sorption in medical devices using polymer materials include the excipient concentration and infusing process. Several experimental procedures have been developed in recent years to determine drug concentrations before and after passing through the medical device.
Recent advances in molecular modeling have improved the rationalization of experimental observations. Along with other advances in analytical methods, this has helped to realize a bottom-up approach to connect chemical structures with desired material properties that limit sorption, additive loss, and drug deterioration, which so far has proven to be a delicate undertaking.
A comprehensive understanding of drug sorption on polymer materials is crucial for designing more effective and safer drug delivery systems, combining experimental observations and advanced molecular modeling approaches. This helps researchers understand adsorption phenomena at both the macroscopic and microscopic level.
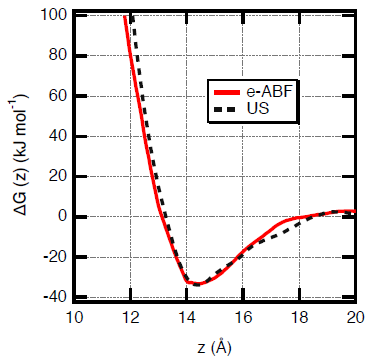
Gibbs free energy profiles calculated during the adsorption of paracetamol onto a polyethylene surface by using the e-ABF and US methods. Image Credit: Sahnoune, M et al., ACS Applied Polymer Materials
The Study
Based on previous work by the authors, the study has proposed a combined and complementary approach to understand the sorption behavior of pharmaceutical drugs on plasticized PVC materials to design better medical devices.
The authors have focused on two commonly used pharmaceutical drugs: paracetamol and diazepam. Firstly, the team designed high-performance liquid chromatography experiments to investigate the plasticizer’s impact on the adsorption of these drugs onto PVC tubings. The second step involved calculating the Gibbs free energy of the process to interpret experimental results.
Advanced molecular simulations were employed in the research to correlate sorption loss in the experiments with the process’s Gibbs free energy. The Gibbs free energy calculations revealed the strength of interactions between the PVC surface and the drugs. A third step involved atomic-scale investigation of the interfacial region and drug mobility.
In the study, three plasticizers whose effects are comparable to PVC without additives were studied: DEHT, TOTM, and DINCH. The partition coefficient of paracetamol and diazepam was used to evaluate the quality of molecular potentials.
The authors noted that the octanol-water partition coefficient is a key property for elucidating information on drug interactions, and reproducing this property provides key evidence on atomistic model quality.
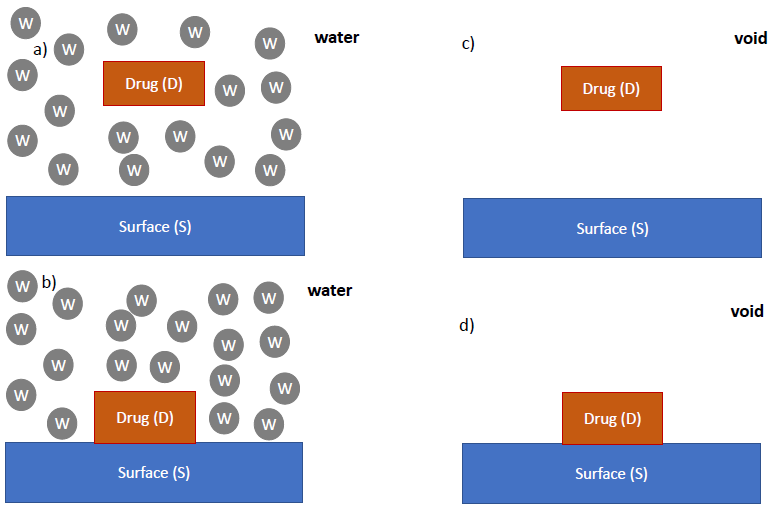
Thermodynamic cycle showing different free energy contributions a) free drug in water, b) adsorbed drug onto the surface in water, c) free drug in the void, d) adsorbed drug in the void where W, D and S refer to the water molecules, drug and surface atoms, respectively. Image Credit: Sahnoune, M et al., ACS Applied Polymer Materials
Study Findings
High-performance liquid chromatography experiments revealed that diazepam strongly adsorbs on plasticized PVC tubings but not on pure PVC tubings. No discernible difference was observed for paracetamol. Simulations revealed that adsorption is thermodynamically favored, with strong Van der Waals interactions between drug and PVC material.
A threshold value of -30 KJ mol-1 was confirmed by comparing experimental observations and simulations. Above this threshold, no significant drug loss to sorption was detected. The authors established that simulations could energetically characterize sorption processes and help with experimental result interpretation.
Interfacial region atomistic descriptions were explored using molecular simulations. These descriptions are not possible by experimentation alone. The authors concluded that the simulations confirmed no absorption, only adsorption, which was evidenced by a simulation time of a hundred nanoseconds. PVC chain, plasticizer, and water density profiles confirmed drug adsorption in line with negative free energy of adsorption values.
The strongest free energy of adsorption values corresponded to the drug’s mobility slowing down slightly both in the direction normal to the surface and the plane parallel to the surface.
In conclusion, the research has demonstrated that this complementary approach can be used to provide characterization and interpretation of adsorption behaviors of drugs on plasticized PVC surfaces. The authors have stated that they hope that this approach could be used in the future to design improved PVC-based medical devices with reduced drug sorption.
Further Reading
Sahnoune, M et al. (2022) Drug Interactions with Plasticized PVCs ACS Applied Polymer Materials [online] pubs.acs.org. Available at: https://pubs.acs.org/doi/10.1021/acsapm.2c00532
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.