Boron (B) is used extensively as a graphitization catalyst in the synthesis of carbonaceous materials used in lithium-ion (Li-ion) battery anodes as it can improve the graphitization degree and reduce the graphitization temperature.
Boron carbide (B4C) clusters are formed when B atoms are inserted into graphite. Additionally, several B atoms can replace the carbon atoms in the graphite since the atomic radius of B is similar to that of carbon, which significantly influences the physicochemical properties, electrochemical performance, and structure of the graphite.
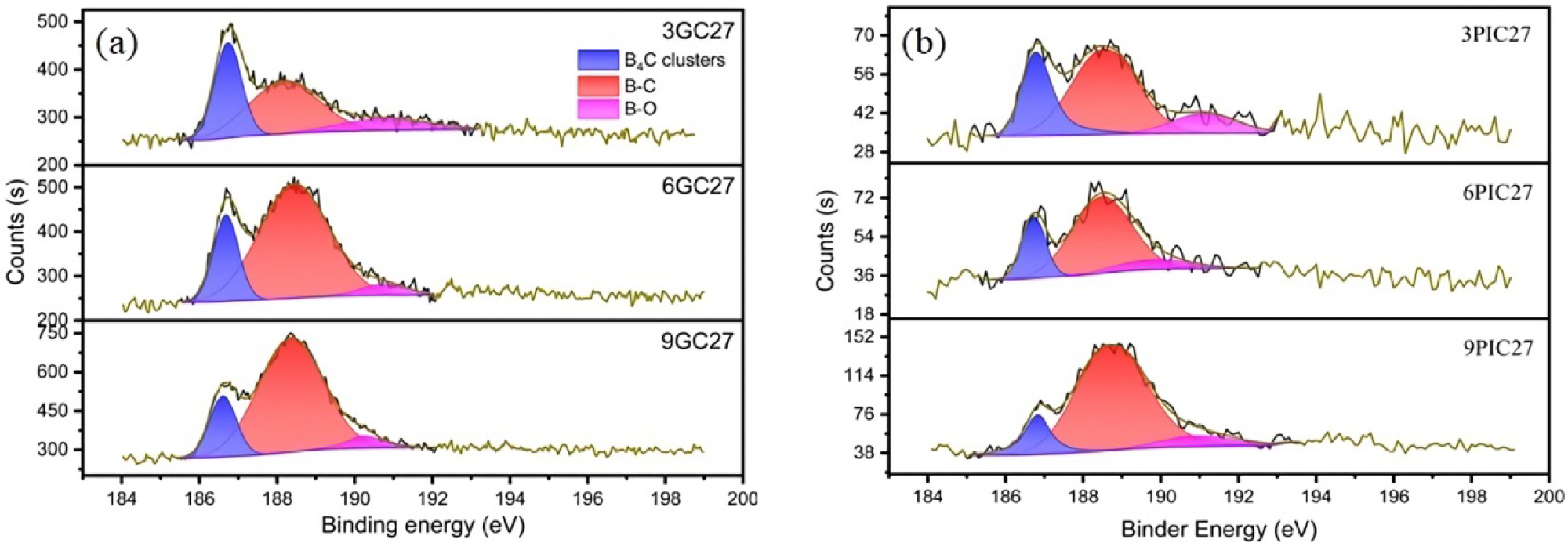
B1s XPS spectra of the boron-doped graphite studied. (a) Boron-doped graphite prepared using needle coke (GC) as raw material; (b) Boron-doped graphite prepared using pitch coke (PIC) as raw material. Image Credit: Bao, C et al., Materials
The reduction in the interlayer spacing is a major structural characteristic of B-doped carbon materials such as graphite. Several studies have demonstrated that B-doped graphite possesses a smaller interlayer spacing compared to ideal graphite.
The substitutional B atoms (sub-B) in the graphite lattice and dissolution–precipitation of carbon atoms are responsible for the decrease in the interlayer spacing. The carbon dissolution–precipitation mechanism is attributed to the exceptional catalytic effects of B, which accelerates the homogeneous graphitization of graphite in a gradual manner. However, the interlayer spacing is increased when the B atoms occupy the interstitial positions in the lattice.
The factors responsible for the smaller interlayer spacing in the B-doped graphite compared to virgin graphite have not been identified until now. However, two mechanisms were considered to understand the effects of sub-B on the graphite interlayer spacing.
The first mechanism was based on the depleted p-electrons between the graphitic layers after B doping which leads to a lower Π-electron density within the graphite layers due to the higher valence state of carbon compared to B and a shorter interlayer distance. The second mechanism was based on the Poisson contraction along the c-axis owing to the expansion along the a-axis. However, these mechanisms were not verified experimentally.
The Study
In this study, researchers synthesized B-doped graphite using B4C powder as a graphitization catalyst through the heat treatment of coke and systematically investigated the sub-B content variation in the carbon lattice after the addition of B4C catalyst and the effects of sub-B atoms on the graphite interlayer spacing.
Pitch coke, needle coke, and B4C powder were used as the starting materials. Initially, needle coke/pitch coke was homogenously mixed with B4C powder and transferred in a graphite crucible placed in the medium-frequency induction graphitizing furnace center.
The furnace was flushed and evacuated with argon gas three times to eliminate moisture and oxygen. The temperature of the furnace was increased to 2700 °C at a 10 °C/min heating rate, and the coke-B4C mixture was annealed under an argon atmosphere for 2 h.
9%, 6%, 3%, and 0% B4C powder was mixed with the needle coke/pitch coke to synthesize eight B-doped graphite samples. The samples synthesized using needle coke were designated as 9GC27, 6GC27, 3GC27, and 0GC27, while the samples synthesized using pitch coke were designated as 9PIC27, 6PIC27, 3PIC27, and 0PIC27 based on their B4C powder concentration.
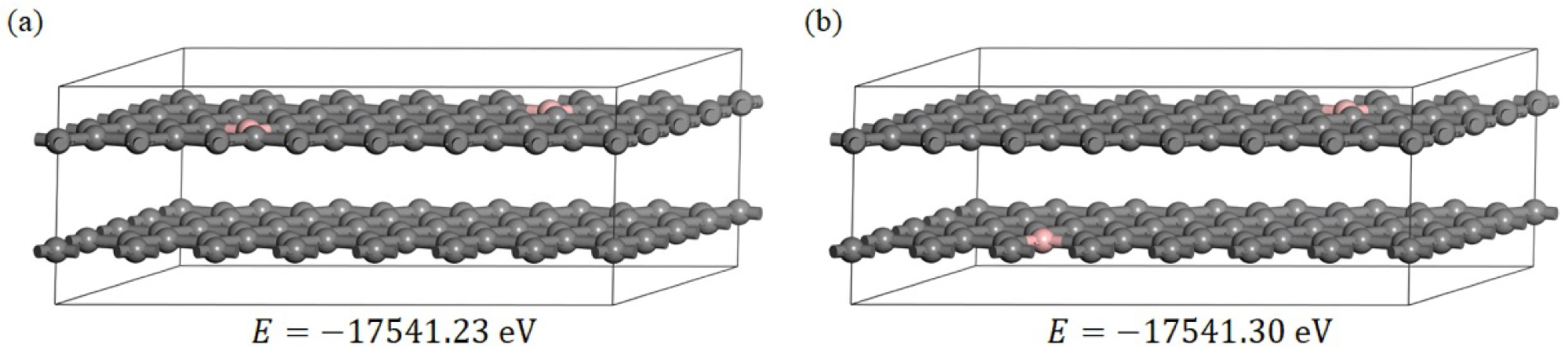
(a,b) two boron-doped graphite models, in which the pink atoms are boron and the grey atoms are carbon; E is the total energy of the models after optimization of the geometry. Image Credit: Bao, C et al., Materials
X-ray photoelectron spectroscopy (XPS) was used to characterize the synthesized samples. The interlayer spacing of the synthesized B-doped graphite samples was measured using the X-ray diffraction (XRD) method and the Bragg equation.
The DMol3 package of Materials Studio was used to perform all density functional theory (DFT) calculations. The interactions formed between the ions and electrons were described using the Linear combination of atomic orbitals. Total energies were calculated by performing Brillouin zone integrations.
The double-numeric quality basis set with polarization functions and the Perdew–Burke–Ernzerhof (PBE) model under the generalized gradient approximation were used to evaluate the electron exchange-correlation interactions.
A periodic B-doped graphite supercell structure was established to understand the sub-B-induced interlayer spacing reduction mechanism. The B mass ratio was set at 1.78% based on the experimental results Two types of B-doped graphite models were optimized and the DFT calculations of the Poisson’s ratio, electrostatic surface potential, total charge density, Mulliken partial charges, and partial density of states were performed on these structures.
Observations
Researchers successfully synthesized B-doped graphite using coke and B4C powder, utilizing the heat treatment method. B-atoms were effectively incorporated into the graphite lattice during heat treatment, leading to a reduction in the graphite interlayer spacing.
The interlayer spacing decreased with the increasing concentration of B atoms in the B-doped graphite lattice. The interlayer spacing values achieved in 9PIC27 and 9GC27 were 0.33537 nm and 0.33545 nm, respectively, which was close to the interlayer spacing value of 0.3354 nm of ideal graphite.
The B doping increased the number of sub-B atoms in the graphite lattice, which broke the electron balance between carbon layers and facilitated the transfer of electrons in small amounts from an adjacent B atom free layer to a B atom containing layer.
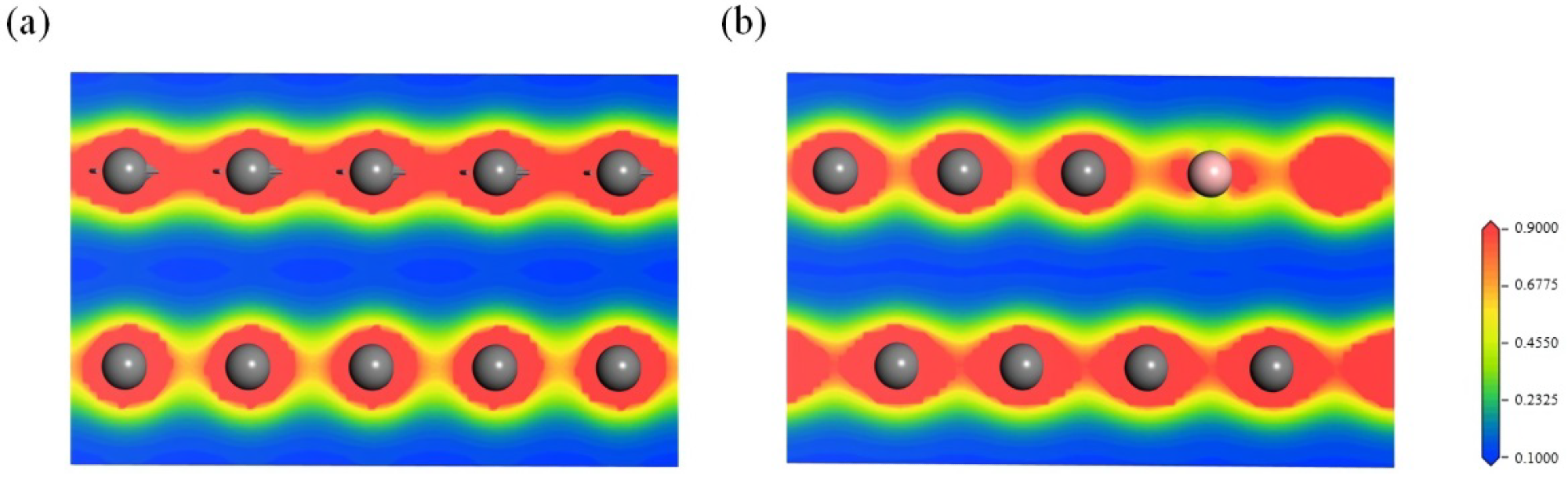
Charge density diagram within the (010) plane of (a) graphite and (b) boron-doped graphite across the boron atoms. Carbon: dark grey, boron: pink. Image Credit: Bao, C et al., Materials
Both the B atom containing layer and its adjacent carbon layer were in an electron-deficient state due to the electron-deficient nature of B atoms and electron transfer from the carbon layer to the B atom containing layer, which increased the electrostatic surface potential of the carbon layers and decreased electron repulsion between the layers.
The attractive forces between the carbon layers and Π electrons in the adjacent layer and the decrease in the Π electron repulsion forces between the carbon layers also played a significant role in decreasing the interlayer spacing.
Taken together, the findings of this study demonstrated that the electron redistribution induced by B doping played the most crucial role in reducing the interlayer spacing in B-doped graphite.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.