Organic synthesis is based on carbon-carbon bond formation. Aldol condensation is one of the key reactions, forming a new β-hydroxy carbonyl compound through the reaction of two carbonyl compounds. This reaction can be carried out under base- or acid-catalyzed conditions. The resultant product is generally an α,β-unsaturated carbonyl compound.
This article presents the results of an experiment involving aldol condensation reaction to gain insights into the characteristics of carbon-carbon bond formations and carbonyl chemistry. This experiment involves the aldol condensation of acetone and p-anisaldehyde (4-methoxybenzaldehyde) under base-catalyzed conditions.
A stepwise sequence is employed to separate the monoaddition (Product A), and the reaction is repeated using Product A to generate the bis-addition (Product B). The Magritek Spinsolve benchtop NMR spectrometer is used in the characterization of the reaction products.
Preparation of 4-(4'-methoxyphenyl)-3-buten-2-one (Product A)
A p-anisaldehyde (1.2mL, 10mmol) is prepared in acetone (15mL) in a 100mL round bottom flask. A magnetic flea is then added and the flask is clamped over a magnetic stirrer. A potassium hydroxide (1.0 g) solution in water (20mL) is prepared in a separate beaker and added gradually to the mixture in the flask with continuous stirring for 20 minutes.
Now, around 40mL of water is added to the reaction mixture to ascertain the precipitation of all products. The resulting solid is filtered by vacuum filtration, followed by water washing. The solid is then dried and recrystalized from ethanol (Figure 1).

Figure 1. Recrystallized 4-(4’-methoxyphenyl) but-3-en-2-one (Product A).
Figure 2 presents the 1H NMR spectrum of p-anisaldehyde, clearly showing the resonances for the aldehyde and oxymethyl proton environments. Inductive effects of the substituents are considered to assign the two remaining resonances that belong to the 1,4-disubstituted benzene ring.
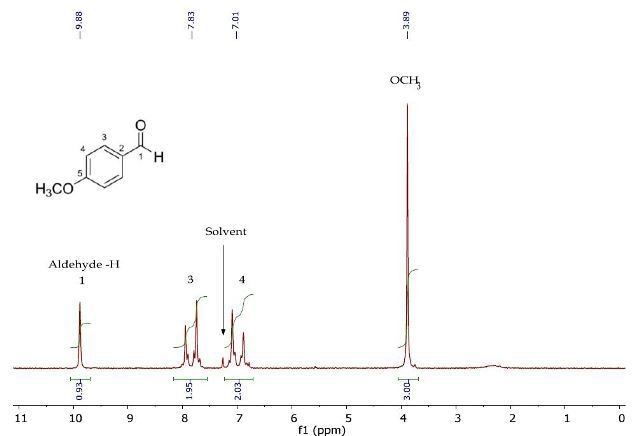
Figure 2. 1H NMR spectrum of p-anisaldehyde in CDCI3.
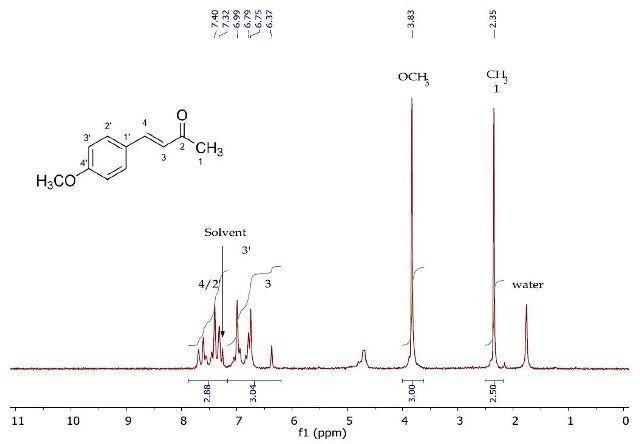
Figure 3. 1H NMR spectrum of 4-(4’-methoxyphenyl)-3-buten-2-one (Product A) in CDCI3.
The 1H NMR spectrum of recrystallized 4-(4'-methoxyphenyl)-3-buten-2-one is presented in Figure 3. The diagnostic methyl resonances at 3.83ppm and 2.35ppm belong to the oxymethyl and terminal methyl at position 1on the aromatic ring, respectively. The remaining resonances between 6.37- 7.69ppm represent the lingering six protons under four chemical conditions. Assigning the chemical shifts and multiplicities of these resonances is a complicated process due to their overlapping. Detailed observation of this region is carried out in the 2D J-resolved and COSY experiments (Figures 4 and 5).
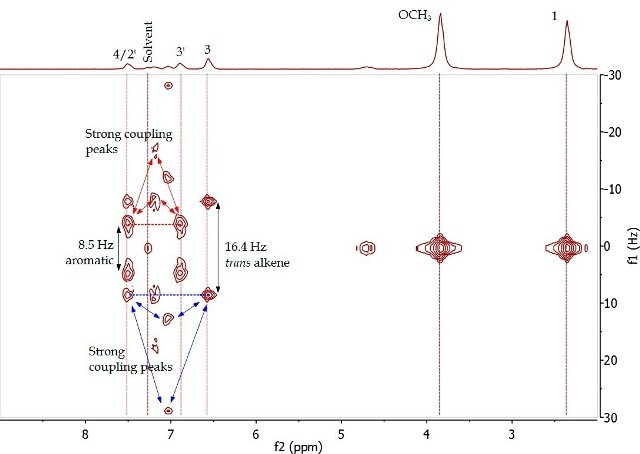
Figure 4. 2D J-resolved spectrum of 4-(4’-methoxyphenyl)-3-buten-2-one (Product A) in CDCI3.
The 2D J-resolved experiment involves mapping of J-coupling constants against the proton chemical shift, thereby allowing accurate measurement of the chemical shifts of proton resonances through f2 dimension examination and their coupling constants in the fl dimension. Spin-coupling partners, typically over 2-4 bonds, are identified in the COSY experiment. Figure 5 presents the COSY of Product A, showing the correlation among the same proton pairs determined in the 2D J-resolved experiment by interpreting cross-peaks in the diagonal of the 2D spectrum. The proximity among these resonances is further validated by these correlations.
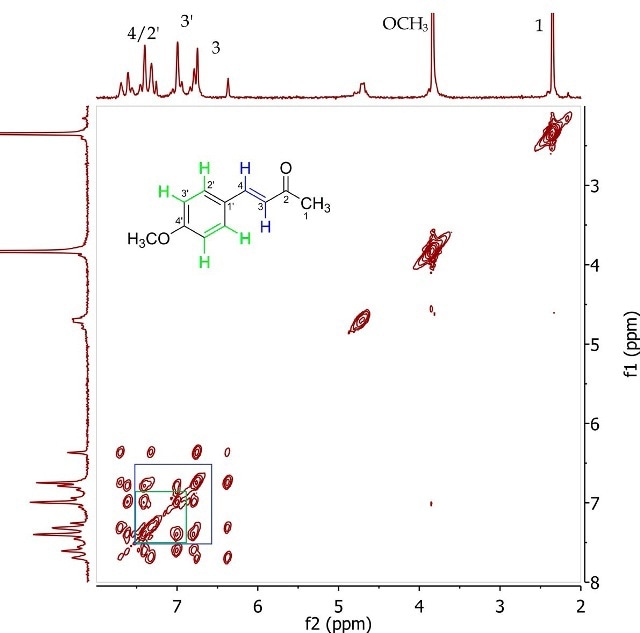
Figure 5. COSY spectrum of 4-(4’-methoxyphenyl)-3-buten-2-one (Product A) in CDCI3.
Preparation of 1,5-bis(4'-methoxyphenyl)-1,4-pentadien-3-one (Product B)
A solution of p-anisaldehyde (0.61mL, 5.1mmol) and freshly recrystallized 4-(4'-methoxyphenyl)-3-buten- 2-one (0.60g, 3.4mmol) in ethanol (15mL) is prepared in a 100mL round bottom flask. A potassium hydroxide (0.8g) solution in water (20mL) is prepared in a separate beaker. The potassium hydroxide solution is then gradually added to the solution in the round bottom flask over two minutes with continuous stirring for 30 minutes. The solid is extracted by vacuum filtration, followed by water washing. The resulting solid is then dried and recrystallized from ethanol (Figure 6).

Figure 6. Recrystallized 1,5-bis(4'-methoxyphenyl)penta-1,4-dien-3-one (Product B).
Figure 7 depicts the 1H NMR spectrum of 1,5-bis(4'-methoxyphenyl)penta-1,4-dien-3-one, confirming the loss of the reactive methyl centre in Product A following the reaction with p-anisaldehyde. In this case also, the 2D J-resolved and COSY experiments are used to interpret the downfield resonances between 6.82-8.02ppm (Figures 8 and 9).
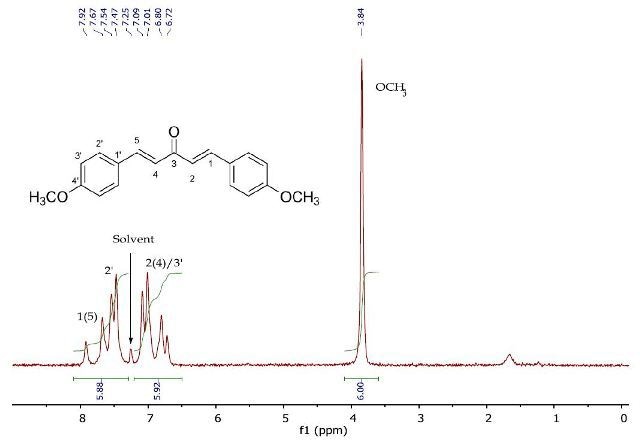
Figure 7. 1H NMR spectrum of 1,5-bis(4'-methoxyphenyl)-1,4-pentadien- 3-one (Product B) in CDCI3.
The J-resolved spectrum (Figure 8) differentiates 8.6Hz doublet and 16.4Hz doublet, centered at 6.90ppm. It also identifies a 16.0 Hz doublet at 7.73ppm and an 8.9Hz doublet at 7.57ppm further downfield. This data is used to determine the two spin systems in the COSY spectrum (Figure 9), one between 6.90-7.57ppm and the other between 6.90-7.73ppm. The alkene protons on C- 1/C-5 and C-2/C-4 can be allocated as 7.73ppm and 6.90ppm, respectively, while the promatic protons on C-2' and C-3' can be assigned as 7.57ppm and 6.90ppm, respectively. The magnitude of the coupling constants of H-1/H-5 and H-2/H-4 confirms the alkene’s E-geometry.
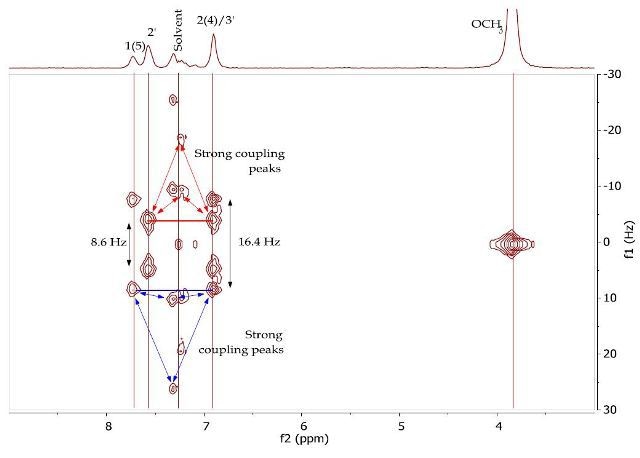
Figure 8. 2D J-resolved spectrum of 1,5-bis(4'-methoxyphenyl)-1,4-pentadien-3-one (Product B) in CDCI3.
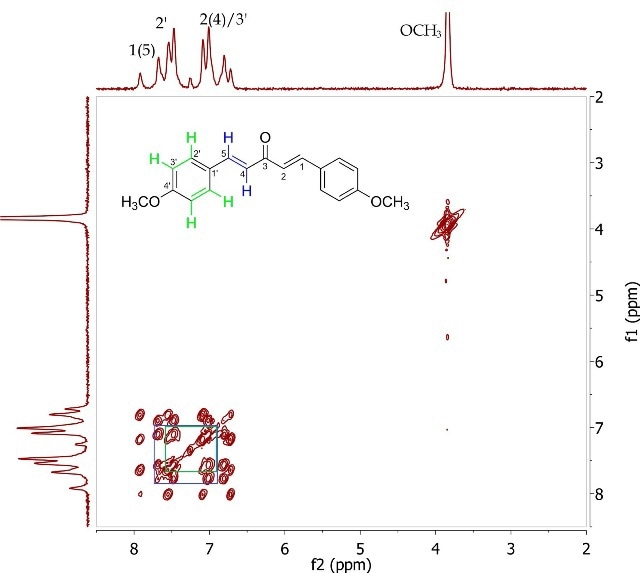
Figure 9. COSY spectrum of 1,5-bis (4'-methoxyphenyl)-1,4-pentadien-3-one (Product B) in CDCI3.
Conclusion
The results demonstrate the advantage of using 1D- and 2D-NMR experiments to gain insights into aldol condensation reaction, a key reaction in synthetic organic chemistry. The 1H NMR spectroscopy can characterize the key features of the chemistry, geometry and structure of the reaction products.
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.