Air, pressure, heat, corrosive agents, and other factors encountered by a lubricant during use can cause chemical changes in the oil. Changes in the oil chemistry can have a negative impact on the working ability of the oil - e.g. when crucial additives are depleted.
Gradually, such chemical reactions can cause build-up of detrimental degradation by-products, such as weak organic acids. Although the analysis of oil chemistry always involves testing oxidation, based on the application, it may also include testing for nitration and sulfation. In specific cases, oils can be also analyzed for the depletion of particular additives, such as ZDDP antiwear package.
Oxidation
The oxidation of oil is caused by the presence of air (oxygen) and heat. Carboxylic acids are formed when atmospheric oxygen reacts with the hydrocarbons in the lubricant. Such acids are weak, but they gradually gain a concentration high enough to cause serious corrosion of machinery parts. This is an inevitable activity that requires monitoring.
To protect the lubricant, antioxidant additives that are oxidized readily before the oxidization of more important components of the oil are added to nearly all of the formulations. Once these additives have depleted, the lubricant properties are negatively impacted. As the oxidation rate varies substantially with temperature and is impacted by contaminants (specifically metals) in the lubricant, the best method to manage oxidation is to keep the oil dry, clean, and cool.
Nitration
Nitration is a major concern in engine oils, specifically natural gas engine oils. Atmospheric nitrogen and oxygen react due to the heat produced and form nitrous oxides (NOx), which in turn react with the lubricant and produce organic nitrates, or are picked up as insoluble or soluble nitrous compounds.
Nitration can lead to a premature thickening of the oil. Common reasons for nitration are; leaking piston seals, improper air-to-fuel ratio, inefficient exhaust of the combustion products, and low operating temperature.
Sulfation
When oxygen, water and sulfur in the base oil or diesel fuel react due to heat, they can form sulfurous compounds, such as sulfur-based acids. These compounds are often discharged via exhaust. However, some compounds may remain and enter into the engine cavity. When the sulfur-based acids react with the base stock of the oil or with the additives in the oil, sulfation occurs.
At lower operating temperatures, such as during start up, these acids are condensed and react more readily with the oil. Sulfation leads to increase in oil viscosity as well as the formation of sludge, varnish, and sedimentation.
Methods of Measuring Oil Chemistry
Viscosity
Although spectroscopy is the only direct technique used to measure oxidation, nitration, and sulfation, Viscosity and impedance measurements are the indirect methods. Viscosity is the measure of a fluid’s resistance to flow and can be increased due to the formation of condensation products as a result of oxidation, nitration, or sulfation of an oil.
Irrespective of the cause, a change in viscosity is a critical parameter to be tested, so viscosity testing is always a part of any lubricant condition monitoring. Several methods such as ASTM D445, ASTM 7279, etc. exist to viscosity, with instrumentation, including the Spectro Scientific Q3050 portable kinematic viscometer, for both lab and in-field testing.
Impedance
Impedance measurement is another indirect method to measure the dielectric properties or conductivity of a fluid. Such properties are highly impacted by a change in the polarity of the hydrocarbons, which is specifically sensitive to oxidation caused by the formation of carboxylic acids. As other contaminants such as water or other degradation by-products can also considerably contribute to impedance change, this test is usually applied for overall trending of the oil condition.
Infrared Spectroscopy
In infrared (IR) spectroscopy, a radiative source, a detector, and a computer are used to analyze the interaction between light and matter. In the IR spectrum, oxidation and nitration products occur as peaks between the wavelengths of 1600 cm-1 and 1800 cm-1. However, sulfation products occur as peaks at a wavelength of around 1120 - 1180 cm-1. As absolute reference standards do not exist for oxidation, nitration, and sulfation, the results are normally compared with those of fresh oil.
For instance, when the engine oil is sampled for a particular time period, if the nitration peak becomes notably more intense around 1650 cm-1, it indicates the occurrence of nitration, probably as a result of improper air-to-fuel ratio. Several test methods exist for both laboratory FTIR measurement and portable field test. ASTM E2412 outlines the standard procedure for FTIR measurement of oxidation, nitration, and sulfation (Figures 1 and 2).
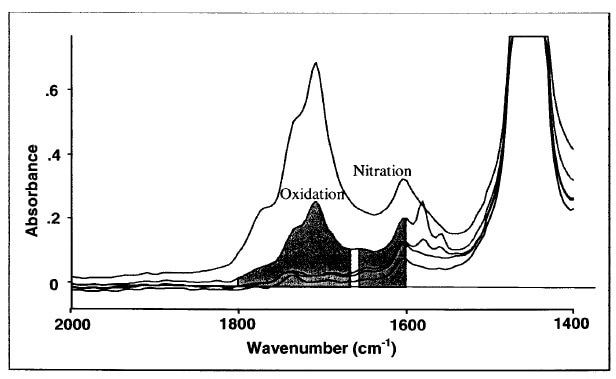
Figure 1. ASTM E2412: Oxidation and nitration measurement in Crankcase Oils.
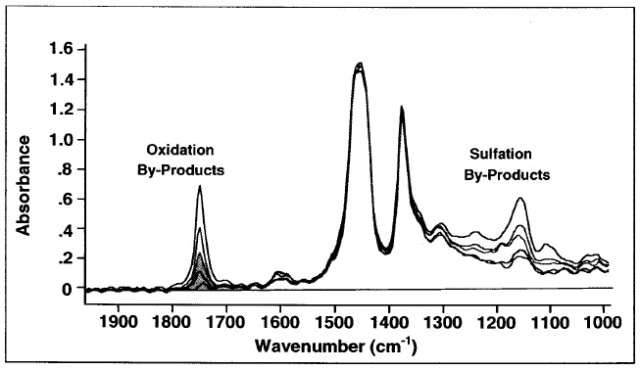
Figure 2. ASTM E2412: Oxidation measurement in EP Fluids.
Specific test methods, such as D7414, D7624, and D7415, have also been determined for oxidation, nitration, and sulfation, respectively. For the purpose of in-field monitoring of oil chemistry, ASTM D7889 employs a grating infrared spectrometer such as the FluidScan (Figure 3) that can be easily operated and does not need a skillful operator.

Figure 3. FluidScan Q1100.
Following are the advantages and disadvantage of the infrared spectroscopy method:
Advantages:
- Low cost per sample after initial equipment purchase
- Enables quick testing
- Highly suitable for direct trending of oil chemistry properties
Disadvantage:
- Mandates costly instrumentation
Conclusion
The analysis of oxidation, nitration, and sulfation in a lubricant oil enables trending of useful service life of the oil, and also signals the failure of engine parts such as piston seals, inappropriate operating conditions, or the use of incorrect lubricant for a specific application.

Figure 4. Thermo Fisher Nicolet FTIR spectrometer.
Although indirect methods such as viscosity and impedance measurement exist to analyze a lubricant’s ability to carry out its functions, the only direct method to check for any oxidation, nitration, or sulfation is infrared spectroscopy. Various FTIR instruments (Figure 4) and techniques exist for average to expert users in a laboratory setup.
The Spectro Scientific FluidScan allows the oil chemistry to be tested either in the field or in the lab using a portable IR spectrometer.

This information has been sourced, reviewed and adapted from materials provided by AMETEK Spectro Scientific.
For more information on this source, please visit AMETEK Spectro Scientific.