|
High nitrogen steels (HNS) are a new class of high alloy martensitic, austenitic or duplex grades with up to 0.9 mass% of N in solid solution. They are applied e.g. to stainless tools and bearings, in chemical engineering and for high strength non-magnetic components.
Dissolving Nitrogen into Steels
In contrast to carbon, nitrogen is a volatile element and therefore requires special measures to be dissolved in the melt. The first one consists of alloying. The Nitrogen solubility increases in the following order of elements Xi = N, C, Si, Al, Ni, Co, Cu/W, Mo, Mn, Cr, Nb, V, Ti, the oblique indicating the change from repulsing to attracting elements. As the solubility product of Nb, V and Ti in austenite is rather low, the Nitrogen solubility of HNS is mainly based on Cr, shifting the majority of alloys into the stainless range. The second measure is to further raise the Nintrogen content of the melt by applying nitrogen pressure. Pressurised electroslag remelting (PESR) is commercially available producing ingots of up to 20 tons in weight. The third measure makes use of the high uptake of Nitrogen in austenite. Respective steel powder is subjected to solid state nitriding and than compacted by hot isostatic pressing (HIP). This allows for the highest Nitrogen content, e.g. 3 mass% in stainless powder metallurgical (PM) tool steels containing e.g. wear resistant NbN nitrides embedded in a martensitic matrix. Measures two and three may lead to Nitrogen contents in the steel, which are above the solubility at temperatures of hardening or solution annealing, if carried out at the partial pressure of nitrogen in air.
Interactions of Alloy Nitrogen
Thus the interaction of alloy nitrogen with the furnace atmosphere or vacuum may differ considerably from carbon and require specific precautions. At first one has to look at the thermodynamic equilibrium between the steel surface and the surrounding atmosphere. Next the kinetics of phase transformations in the whole cross section are of interest to avoid e.g. embrittling precipitates. This is especially important for stainless austenitic steels, which are usually low in carbon but may contain up to 0.9 mass% nitrogen. Finally the expected changes in volume and the resistance to oxidation of HNS during heat treatment have to be considered in comparison to respective carbon grades.
Thermodynamic considerations
Nitrogen Concentration
During manufacturing of high Nitrogen steels the [N] content dissolved in the steel is in equilibrium with the partial pressure pN2 in the atmosphere along ½N2 = [N].
The reaction constant of this equation is related to the free energy
 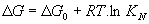
which becomes zero in case of equilibrium leading to
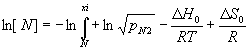
Activity of Nitrogen
The activity of nitrogen aN is expressed by the concentration [N] times the activity coefficient of nitrogen in a steel alloyed with repulsing and attracting elements Xi as mentioned above. The latter are described by negative interaction parameters changing the sign of the first term to positive. Thus elements like Cr and Mn raise [N] as does pN2. For these elements the standard heat of solution ΔH0, is negative causing a decrease of [N] if the temperature T in the third term is raised. The final term contains the change of standard entropy ΔS, and the gas constant R.
The main difference of equation (1) in respect to [C] of carbon grades is the pronounced pressure dependence of [N]. While stainless carbon steels are satisfactorily heat treated in vacuum furnaces, an effusion of Nitrogen is to be expected for high Nitrogen steels. If the latter are treated in pure nitrogen of 1 bar pressure the dissociation of N2 is low up to 700°C which amounts to a shielding atmosphere. Above 900°C N2 becomes increasingly thermally dissociated and as terms one to three of (1) are set by a given steel and temperature, effusion or infusion of Nitrogen is liable to occur except for some narrow range of parameters. Therefore the safest way to proceed is to calculate the required PN2 in equilibrium with [N] of a given steel at a required temperature. However, this implies that for pN2 > 1 bar a pressure chamber is used, while at pN2 < 1 bar a dilution with argon at a total pressure of 1 bar is feasible as well. The lower the temperature of hardening or solution annealing, the less pressure has to be applied.
The Effect of Oxygen on Carbon and Nitrogen Containing Steels
Even at a small activity of oxygen within the furnace atmosphere, steels of high Cr content tend to oxidise during heat treatment. In stainless carbon grades, carbon is also oxidised leading to decarburisation and fissures in the scale caused by carbon monoxide molecules on their way out. Not so in high Nitrogen steels, in which the scale goes unharmed and adheres well to the steel surface. In a near surface zone, though, the Nitrogen content was found above that of the available pN2 pointing to a higher aN at the scale/steel interface, which may be supported by a displacement of Nitrogen from the oxidised layer of the steel inwardly. An example is given in Table 1 for solution annealing in air, respectively in nitrogen of equivalent pN2 = 0.8 bar. While the latter treatment meets the calculated N content except for a disturbed surface film, the former exceeds the calculation considerably.
Solution Nitriding
The equilibrium between pure N2 and a stainless steel surface is also used to intentionally dissolve [N] in the outer case of near net shape parts. This new heat treatment is called solution nitriding, which is done at a temperature of 1100±50°C and a pressure of 0.1 < pN2 < 3 bar. After ~24 hours a case depth of ~3 mm is obtained. The aim of solution nitriding is to transform the case of conventional stainless steels into high Nitrogen steel. Depending on the type of steel a hard martensitic case is formed during quenching (case hardening with nitrogen or a high strength, yet ductile austenitic one. IPSEN International introduced the trade names SolNit-M and SolNit-A and the Gerster AG - Switzerland is offering the new process commercially.
Kinetic considerations
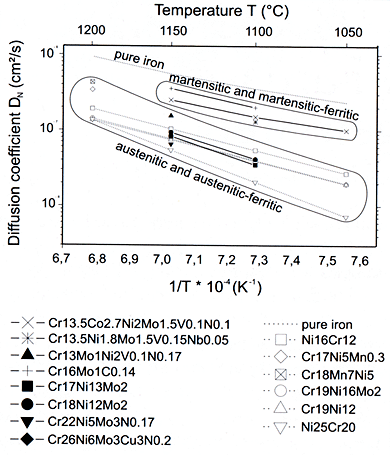
As shown above, high nitrogen steels require a high Cr content besides other elements to influence the constitution and properties. In high alloy steels diffusion is retarded. Fig. 2 summarises the effect of temperature and alloy content of different steels on the coefficient of nitrogen diffusion. Interstitial nitrogen is quicker than substitutional elements by several orders of magnitude.
|
|
|
Figure 1. Effect of temperature on the diffusion co-efficient of Nitrogen in pure iron and stainless steels.
|
The Importance of Heating
During heating the dissolution of precipitates and other phase transformations are slow in high alloy steels and require a sufficient soaking time. One is well aware of the phenomenon as far as martensitic steels of high Cr and C content are concerned. Little difference is expected for respective high nitrogen steels in which Cr nitrides instead of Cr carbides have to be dissolved prior to hardening. Stainless austenitic and duplex steels are commonly of low interstitial content, though, and in this respect high nitrogen steels are quite different. They take longer to reach a homogeneous distribution of e.g. Cr atoms after the dissolution of nitrides, which is a prerequisite of a high resistance to corrosion.
The Importance of Cooling
During cooling the high interstitial content of high nitrogen steels tends to provoke a precipitation of nitrides if a critical cooling time t8/5 is exceeded referring to the duration of cooling from 800 to 500°C. If all nitrides are dissolved during austenitisation no nuclei for precipitation during cooling are left and a decoration of grain boundaries will occur first, followed by a discontinuous growth of M2N lamellae into austenitic grains. The resulting microstructure is termed “nitrogen pearlite”. For the sake of toughness and corrosion resistance nitrides have to be subdued by quenching.
Scale formation
The interaction of scale and interstitial elements was discussed earlier. In stainless steel dense chromium oxide forms a barrier against the migration of oxygen and metal ions and retards the oxidation of carbon and nitrogen grades. Comparing both types of stainless steel, nitrogen seems to improve the resistance to scaling. Of martensitic, creep resistant steels with (mass%) Cr9W2Mo0.5V0.2Nb and 0.045 respectively 0.168 N the latter showed a considerably reduced weight gain in air between 500 and 900°C and up to 104 hours. An exchange of (mass%) 0.5C + 0.5 N by 0.9 N in stainless austenitic steels for exhaust valves enhanced the resistance to oxidation at 850°C and ~500 hours. Therefore material loss by scaling during heat treatment of heavy components in air is impeded by Cr and apparently further retarded by N.
Distortion
The term distortion comprises a change of shape or of size and size stability. The first part depends mainly on external parameters like shape, taking, loading, supporting and quenching of the work pieces and little difference is expected between stainless carbon or nitrogen grades. The size change, however, relies on internal, microstructural and thermal changes of volume during heat treatment. The thermal ones are size dependent and again little difference is anticipated between stainless carbon and nitrogen grades considering the relatively low heating and cooling rates of high alloy steels. Volume changes by phase transformation are bound to be different, though.
Martensitic Stainless Steels
Looking at martensitic stainless steels an exchange of carbon by nitrogen enhances short range atomic ordering of Cr atoms and stabilises the austenitic phase, which results in a higher content of retained austenite (RA) and a smaller size of high Nitrogen steels after hardening. Therefore deep freezing and tempering in the range of secondary hardening at about 450°C is required to reduce RA. The highest degree of ordering and RA content is met in steels with C+N like in PESR steel Cr15Mo1C0.3N0.4 used e.g. for stainless bearings. After tempering at 450°C martensitic high nitrogen steels are prone to reveal a good size stability during service at room or slightly elevated temperature. As the nitride precipitates responsible for secondary hardening are not enriched in Cr the corrosion resistance is retained. In contrast respective carbon grades are restricted to lower tempering temperatures and small volume changes by retarded precipitation or RA transformation during service may impair the size stability. Austenitic stainless steels are commonly of low interstitial content. Respective high Nitrogen Steels contain up to 0.9 mass% N, though, the major part of which is precipitated as nitrides during slow cooling after hot working. The PESR steel Cr16Mn14Mo3N0.9 is an example and used for high strength, non-magnetic retaining rings holding the wiring of electric power generators. Solution annealing entails a slight increase of volume and therefore should be carried out before final machining, which is a common procedure, though.
Conclusions
Compared to conventional stainless steels, high nitrogen grades behave differently during heat treatment.
a) Nitriding and denitriding may occur, if the partial pressure pN2 of the furnace atmosphere is not in equilibrium with the alloy concentration of the steel at a given temperature.
b) An embrittling precipitation of nitrides is impending if a critical cooling time is exceeded.
c) The rate of scaling was shown to be lower for high nitrogen steels. In contrast to the commonly encountered decarburisation, nitriding was observed in the steel surface adjacent to the oxide layer after high temperature treatment in air.
d) In martensitic steels distortion is influenced by a higher content of retained austenite in HNS. The size stability of HNS is enhanced by secondary hardening.
|