In this interview, AZoM speaks with Greg Gargus, Analytical Chemist and ChemE Sterilization Account Specialist; and Ryan Lerud, PhD, Product Manager, Process Insights about the recent advances in hydrogen peroxide sterilant vapor monitoring.
How did Guided Wave enter the vapor sterilization market?
In 1983, Guided Wave was an industry pioneer when it delivered the first commercially available fiber-optic-based near-infrared (NIR) spectrometers for in-situ process analysis. Soon after, it developed a H2O2 and H2O vapor optical monitoring unit using NIR wavelengths.
Researchers at Baxter and Steris (Amsco) partnered to develop a precalibrated sterilization cycle development tool for concentration mapping within a sterilization chamber or isolator. This collaboration resulted in a 1998 scientific article (Adams, Dave, et al. "Calibration of a near-infrared (NIR) H2O2 vapor monitor." Pharmaceutical engineering 18.4). This illustrated how a NIR calibration for hydrogen peroxide and water vapor was developed and cross-validated. A patent on the optical NIR method was also issued.
As a result of the collaboration, a sensitive and stable NIR spectrometer was developed, and a commercial unit called the Hydrogen Peroxide Vapor Monitor (HPVM) became available. The HPVM provided a turn-key solution for monitoring hydrogen peroxide sterilant loads.

The original HPVM and orginal 25 cm vapor probe. Image Credit: Guided Wave
What is special about near-infrared (NIR) light?
NIR light helps monitor sterilants' molecular vibrations using their specific light absorptions. As a result, using NIR optical methods, fast and direct chemical measurements are made without the need for indirect sensors, wet chemical methods, or other time-consuming procedures.
Why monitor sterilant vapor concentrations with NIR Spectroscopy?
Near-infrared spectroscopy provides a sterilization monitoring solution that operates in a positive and negative atmosphere. Because NIR spectroscopy uses light to monitor the chemical concentrations, the Vaporized Hydrogen Peroxide Analyzer can monitor sterilant levels under non-condensing conditions. The number of sterilant and moisture molecules in the NIR optical beam causes the measurement signal.
What other technologies are used for sterilization monitoring?
Electrochemical sensors are also used for sterilization monitoring. During the 1990s, the use of H2O2 as a sterilant occurred mainly in atmospheric isolators. Electrochemical sensors worked well for this type of vapor measurement. These devices were a good choice at a lower price point because the robust measurement advantages of NIR spectroscopy were not necessary. However, electrochemical sensors cannot operate in a vacuum and can also be damaged or poisoned by high sterilant concentrations.
What changes have you seen in the market for sterilization measurements?
The need for low-temperature sterilization for medical devices and other challenging substances has increased in the last two decades. The development of vacuum chambers to enhance sterilant drive into packaging and product surfaces followed. However, popular for atmospheric isolators, electrochemical sensors do not work in a vacuum or in atmospheres where pressure changes. Most electrochemical sensors also do not measure moisture.
We have also seen the need to monitor other sterilant gases, such as ethylene oxide and nitrous oxide, using optical spectroscopy.
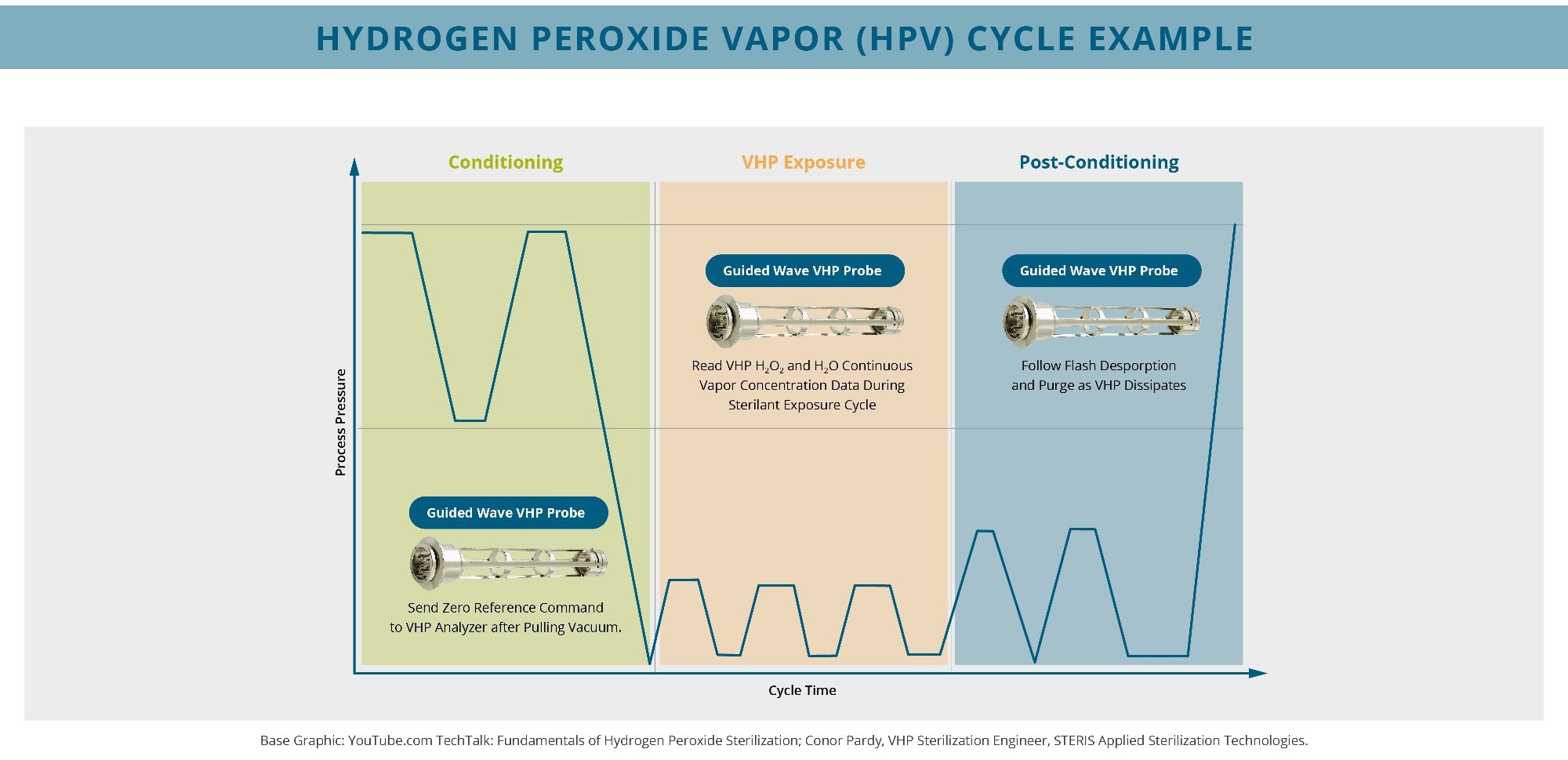
This chart illustrates a typical low-temperature, vacuum sterilization with sterilant pulse injection. The Guided Wave HPV analyzer provides high-fidelity monitoring of both H2O2 and H2O vapor concentrations during pressure changes. Image Credit: Guided Wave
How has the NIR Sterilant Analyzer evolved to meet these changing market demands since its introduction over 25 years ago?
The original HPVM was a unique fiber-optic-array spectrometer for superior direct sterilant measurement. This complete NIR analytical system comprised three main components:
- The NIR spectrometer
- A pair of fiber optic cables for each probe
- The sample interface, in this case, the G-SST vapor probe.
The spectrometer transmits the source light and quantifies the sterilant vapor-modified light, while the specialized fiber optic cables enable localized vapor probe placement. Longer fiber optic cables have also been used for monitoring a network of biosafety environments for decontamination.
The next-generation HPV Analyzer includes a dual-beam, multiwavelength NIR photometer with two independent sampling points. The design enables factory wavelength changes, so other sterilant vapors, such as ethylene and nitrous oxide, are also monitored. The dual-channel systems are particularly useful for R&D, cycle development, diagnostics, and differential process monitoring.
The HPV Analyzer is the best choice for end users who need a vapor probe placed at various locations within a chamber. The redesigns of the remote vapor probe have improved durability under changing vacuum conditions. The HPV ClearView db filter photometer and remote vapor probe will continue to be commercially available.

Image Credit: Guided Wave

The ClearView db HPV analyzer and a modern folded path 50 cm vapor probe. Image Credit: Guided Wave
In 2022, the new LED-HPV Analyzer was recently developed for monitoring through an isolator wall. The analyzer system was simplified by removing the fiber optic cable and putting the analyzer directly on the probe while also reducing acquisition costs. This had the added benefit of improving integration with a sterilization chamber or whole-room decontamination. It employs long-lasting LED light sources, ensuring long-term, stable performance with minimal maintenance and power requirements. The electronics are located within an enclosure directly on the atmospheric end of the optical probe, creating a complete and compact total solution with more installation flexibility for chamber wall implementation.
Optional capabilities for the LED HPV Analyzer are positioned to address new regulatory goals for better sterilant vapor control, leading to release testing.
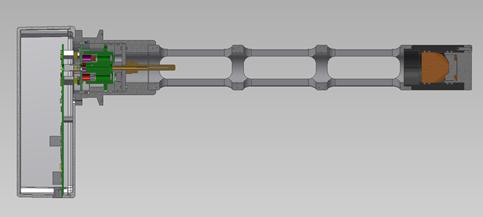
New LED-HPV Analyzer with simpler design for in-situ sterilization monitoring. Image Credit: Guided Wave
Is the LED-HPV Analyzer used for control purposes or point monitoring?
A Guided Wave LED-HPV Analyzer can be used for monitoring and control purposes. New Forge Technologies delivers sterilization process control using NIR in its Bier Resistometer.
Dr. Matt Schurman and his team of engineers and scientists found the ISO protocol was not being rigorously implemented for instantaneous burst exposure to HPV Sterilant Vapor. The Bier Resistometer is a biological (BI) and chemical (CI) Test and Standards Chamber.
New Forge developed an innovative Bier Resistometer with a built-in HPV Analyzer for controlling sterilant injection and providing precise and instantaneous HPV exposure. The process diagram below illustrates how New Forge achieves precise HPV concentration for a standardized sterilant exposure for (BI) and (CI) indicator strips.
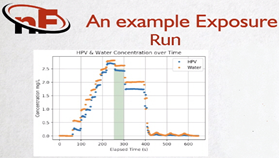
HPV buildup phase followed by load exposure in new Forge Bier Resistometer. Image Credit: Guided Wave
How does New LED-HPV Analyzer complement rapid biological indicators?
Biological indicators (BI) have been the final go/no go of sterilization validation testing. By placing biological indicators in the sterilization chamber with the load (product to be sterilized), technicians can verify that a kill dose of sterilant was received to regulatory standards. However, BIs require time to develop an answer, and the load is quarantined during this period. The LED-HPV Analyzer was developed to complement and advance release testing technology. A basis for better control of the sterilant concentration is emerging by providing real-time monitoring.
In the past, if a biological indicator showed that a kill dose was not received, the data produced by the Hydrogen Peroxide Vapor Analyzer could be used to help explain what went wrong with the sterilization cycle. By combining biological indicators with NIR, engineers designing the next generation of pallet-to-tabletop-sized sterilizers can reduce the time to market for new products. New ambitious regulatory goals for automated release testing call for the sterilization chambers to have real-time process chemical load validation.
Are there any new regulatory releases impacting this business?
In August 2022, ISO22441 was released, which included sterilization of healthcare products, low-temperature vaporized hydrogen peroxide, and requirements for the development, validation, and routine control of a sterilization process for medical devices. This mandates using a tool such as a NIR optical vapor monitoring for validating sterilant loading and correlating sterility. The tools and the sterilizer designs and controls are moving toward automated load release testing. A high-fidelity HPV real-time analyzer is a critical component toward realizing these goals. The HPV LED Analyzer has other capabilities that are useful for the optimization of sterilant vapor exposures.
Does the LED-HPV measure anything other than H2O2 and H2O vapor concentration?
The LED-HPV Analyzer includes an RTD temperature sensor. This enables the direct calculation of Relative Humidity (RH) and Relative Saturation (RS) inside the chamber. The ability to independently calculate relative saturation, in addition to hydrogen peroxide and relative humidity, enables improved process control and exposure times while avoiding condensation inside the chamber.
The data is output over RS232 or RS485 to a PLC or other data logger. With a minimum scan speed of one second, the LED-HPV Analyzer provides real-time data for process control. With the addition of a sanitary fitting, the sample interface can be mounted in any sterilization chamber or isolator with an available >50 mm diameter port.
When do you expect the release of the LED-HPV Analyzer?
We anticipate the introduction of the LED-HPV Analyzer in Q1 of 2023. This will provide a valuable tool for monitoring and controlling sterilant vapor.
About Ryan Lerud and Greg Gargus
Ryan Lerud
As Product Manager/Application Specialist, Dr. Ryan Lerud’s primary focus is application development, providing pre- and post-sales support, including technical presentations, equipment demonstrations, system troubleshooting, and customer training.
Dr. Lerud has over 10 years of industry experience in hardware/software development, field service and installation, instrument repair and maintenance, and new product development. He received his Ph.D. in Applied Physics from Portland State University, Portland Oregon. He also earned a Bachelor of Science Degree in Physics from Portland State University, and an Associate’s Degree in Mechanical Engineering Technology from Mt. Hood Community College, Oregon.
Before joining Process Insights, Dr. Lerud worked as an Application Scientist for an Oregon-based manufacturer of BioScience equipment. While employed there, his work primarily focused on spectroscopic and gas-sensing technologies for various instruments.
Dr. Lerud is a recognized expert in the spectroscopic analysis of fresh produce and has several publications dealing with the multivariate analysis of spectral data and analyzer hardware design. He is an elected delegate-at-large for the Council for Near Infrared Spectroscopy, serving in that capacity until 2020.
Greg Gargus
Mr Greg Gargus is founder and President of Prochem Scientific. Since 1984 he has assisted with the development then business adoption of several new chemical measurement technologies. These include utilization of NIR in pharma operations, novel catalyst screening systems, process chromatography, applied industrial Raman analysis, and an environmental analyzer that won a prestigious R&D 100 award.
He holds a BS in Analytical Chemistry from Penn State University. A Masters degree was awarded for the first interdisciplinary engineering program at Penn State also. Professor Laurent Beaudry privately mentored Mr Gargus for 7 years in the discipline of technical marketing, product development considerations of moving technologies from lab bench to process and the challenge of defining technology trends. Mr Gargus also teaches a course on Innovation in Kenya East Africa periodically.
Mr. Greg Gargus has worked with Guided Wave brands since 1984 and has helped shape applications, product development along with business development especially in applied process implementation. In 2020 Process Insights appointed him as Sterilization Account Specialist given the years of experience in this vapor sterilizaiton market.

This information has been sourced, reviewed and adapted from materials provided by Guided Wave.
For more information on this source, please visit Guided Wave.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.