Introduction Hydroxyapatite (Ca10(PO4)6(OH)2, HAp) has been of great interest as a material for surgical implants due to its great biocompatibility with living tissues [1]. Unfortunately, in immersion test, large size defects appeared during dissolution so that degradation occurred and the fracture toughness of the HAp ceramics did not exceed the value of 1 MPa.m1/2. Research has been focused on the improvement of mechanical performance of synthetic Hap [5, 6] and the addition of a secondary phase as sintering additive to improve densification [7]. In this work, defects formation especially at the initial stage of dissolution and the method to reduce the initial stage defects or pores at the grain boundaries were examined. Mechanical properties of synthetic HAp without altering their biocompatibility were also evaluated by introducing small quantities of silicate glass powders as sintering additives. Experimental Procedure Commercially obtained hydroxyapatite powders (Junsei, Ca/P ratio 1.619) are used as raw materials in the experiment. Calcium silicate glass powders are added to HAp powder as sintering additives (GR-HAp). The mixture was wet milled for 8 h with methanol as a suspension medium and dried for 24 h in an oven at 100°C, followed by sieving to particle size <75 μm in order to obtain homogeneous free-flowing mixed granules. HAp and GR-HAp powders were uniaxially compacted and subsequently cold isostatic pressed (CIP) at 220 MPa. The obtained compacts were sintered at 1200° for 2 h in air under moisture protection that consisted of bubbling air through water. The density of the sintered bodies was measured using the Archimedes technique. After sintering, the disks were polished to smoothness using 1μm diamond paste. All of the dissolution tests were conducted in Tris solution (SBF) with deionized water for 1 to 7 days. The SBF solution was prepared according to the Kokubo’s Formulation [8], as buffered solution (pH of 7.4 at 37℃). At the end of immersion all samples were washed with distilled water and acetone then dried at 50° for 24 h. After the immersion test of samples, the microstructure was observed by scanning electron microscope (Philips XL30 S FEG) and also transmission electron microscope (JEM-2010, JEOL). The phase composition of all samples was analyzed by Fourier transformed infrared spectroscopy (FT-IR). Fracture toughness, KIC was determined on 1μm polished samples using Vickers microhardness tester (HM-112, Akashi). KIC values were calculated using the following equation [9]: KIC = 0.016 × (E/H)0.5 × (P/c1.5) where E, Young’s modulus, H, the hardness, P, the load applied and c is the crack length. Results and Discussion Crystal structures of the samples sintered at 1200°C were confirmed by XRD analysis. All samples showed only the HAp peaks consistent with the values of Ca/P ratio and nearly full densification having 97-98% of the theoretical. But it has been well known that small amounts of the HAp would be decomposed to α-tricalcium phosphate (α-TCP) at grain boundaries [6]. When the glass powder was added, FT-IR spectrum (Figure 1 a), SG-HA) showed little changes of crystal structures because the absorption patterns of HAp, especially OH band of 3570 and 630 cm-1, were slightly decreased. It is more obvious in TEM micrographs. Figure 2 a) shows the changes of grain boundary morphologies of HAp specimen with immersion time of 1day. Although the polished surface was smooth and had a relatively low density of surface defects as shown in Figure 3 a), the nano-size defect was initiated at the grain boundaries at the initial stage of dissolution. These grain boundary defects grow to large scale according to immersion time so that after 3days the surface damage was more disseminated producing a larger and higher density of pitting as shown in Figure 3 a). 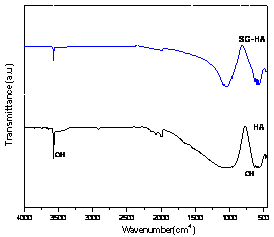
(a) 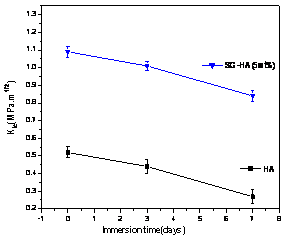
(b) Figure1. (a) FT-IR spectrum and (b) fracture toughness of HAp and glass added GR-HAp. But in the GR-HAp specimens significant grain boundary dissolution was not observed after 1 day of immersion time. It was probably due to the absence of grain boundary secondary phase as seen in Figure 2 b). Even after 3days of immersion, the surfaces became less rough than pure HAp which surface was more considerably damaged. As a result, the addition of 5 mass% calcium silicate glass significantly improved the fracture toughness of HAp to the range of 0.8-1.1MPa.m1/2, which was double than that of pure HAp as shown in Figure 1 b). 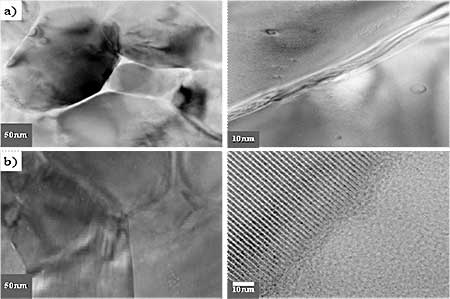
Figure 2. TEM micrographs of a) hydroxyapatite(HAp) and b) glass added hydroxyapatite (GR-HAp) after immersion test of 1 day. 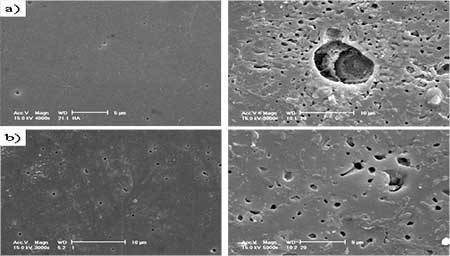
Figure 3. SEM images of a) hydroxyapatite(HAp) and b) glass added hydroxyapatite(GR-HAp) after immersion test of 1 day and 3 days. Conclusions The effect of silicate glass addition as sintering additive to HAp and their densification, microstructural development and mechanical properties were studied. 1) Result of FT-IR analyses showed that when the calcium silicate glass was added, crystal structure was changed a little showing the decrease of OH bands. TEM results showed that secondary phase around grain boundaries was significantly decreased. 2) At the initial stage of immersion test, nano-size defects initiated at grain boundaries and subsequently particles loosing occurred in the polished surface. 3) When calcium silicate glass was added as sintering additive, surface dissolution and particle loosing decreased and the mechanical properties significantly improved. Acknowledgements This work was supported by KOSEF Grant (R01-2005-000-10617-0). References 1. L.L. Hench, “Bioceramics”, J. Am. Ceram. Soc., 81 (7) (1998) 1705-28. 2. C. Rey, “Calcium phosphate biomaterials and bone mineral: Differences in composition, structure and properties,” Biomaterials, 11 (BIMAT 89) (1990) 13-15. 3. P. Ducheyne and Q. Qiu., “Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function”, Biomaterials, 20 (1999) 2287-303. 4. A. Ravaglioli and A. Krajewski., Bioceramics: Materials, properties, and application, pp. 156-197, Chapman& Hall, London (1992). 5. D.M. Liu and H.M. Chou., “Formation of a new bioactive glass ceramics,” J of Mat. Sci.: Materials in medicine, 5 (1994) 7-10. 6. D.S. Seo, J.K. Lee, H. Kim and J. Lannutti, “Dissolution on the surface of calcium phosphate ceramics in water”, J. Ceram. Soc. of Japan, supplement 112-1, Pacrim5 special issue, 112 [5] (2004) S829-S834. 7. W. Suchanek and M. Yoshimura., “Hydroxyapatite ceramics with selected sintering additives”, Biomaterials, 18 (1997) 923-33. 8. T. Kokubo, T. Yamamura, L. L. Hench and J. Wilson., CRC handbook of bioactive ceramics, Vol. 1, p.41, CRC Press; Boca raton (FL) (1990). 9. S.H.Youn, J.J. Kim, G.C. Kim, K.H. Hwang, J.K. Lee and H. Kim., “Characteristics of Liquid Phase Sintered Hydroxy-Apatite”, Archives of Bioceramics Research, 5 (2005) 352-355. Contact Details |