Scientists have always been intrigued by how biominerals, such as bone, teeth, and seashells are formed. A team of researchers from the University of Wisconsin-Madison has illustrated the experimental observations of the earliest stages of nacre formation in a mollusk for the first time.
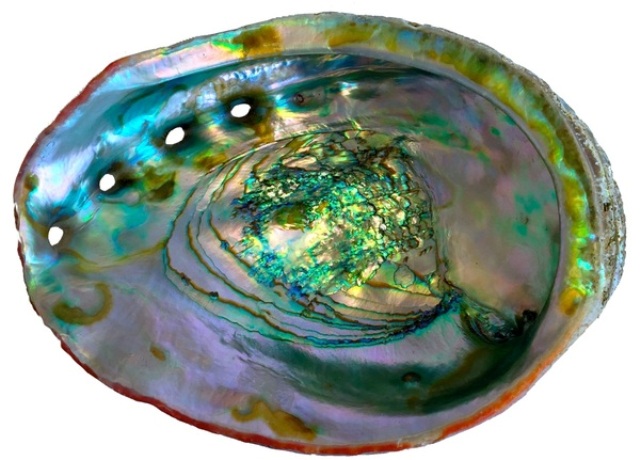 The red abalone makes the lustrous but hard-as-nails nacre lining of its shell by changing the atomic structure of amorphous calcium carbonate. Also known as mother-of-pearl, nacre has been worked by humans for millennia to make jewelry and fancy inlay for furniture and musical instruments. (Photo: Pupa Gilbert)
The red abalone makes the lustrous but hard-as-nails nacre lining of its shell by changing the atomic structure of amorphous calcium carbonate. Also known as mother-of-pearl, nacre has been worked by humans for millennia to make jewelry and fancy inlay for furniture and musical instruments. (Photo: Pupa Gilbert)
The nacre, also commonly known as mother-of-pearl, is an intriguing biomineral. The shells of some mollusks are lined with a silky, iridescent, harder-than-rock composite, which also forms a coating on pearls. Nacre has been widely exploited for centuries to manufacture numerous items like buttons, architectural tile, tooth implants, and inlay for musical instruments and furniture.
Despite many years of study, the way that the mollusks first deposited the nacre has remained a mystery, until this latest research by the Wisconsin team led by physics Professor Pupa Gilbert. The team chose to observe the red abalone, a marine mollusk possessing a domed shell lined with nacre for its research. With the aid of the U.S. Department of Energy's Advanced Light Source at the Lawrence Berkeley National Laboratory, the team was able to illustrate the initial phases of nacre formation at the atomic scale as well as at the nanometer scale. They presented their findings in the Journal of the American Chemical Society.
"People have been trying to understand if nacre had an amorphous calcium carbonate precursor for a long time," explains Gilbert, an expert on the formation of biomineral, indicating that non-crystalline calcium carbonate forms the foundation for nacre formation. "There is just a tiny amount of amorphous material. It is very hard to catch it before it transforms."
Gilbert’s team directly observed the chemical conversion of amorphous calcium carbonate into the aragonite mineral using a combination of spectro-microscopy and the synchrotron radiation produced by the Advanced Light Source. The resulting aragonite mineral then grows into nacre through the deposition of microscopic polygonal aragonite tablets in the same manner as brickwork, thereby strengthening the shiny and tough biomaterial.
"We could only capture it in a handful of pixels, about 20 nm in size, at the surface of forming nacre tablets," says Gilbert about the manner in which the mollusk deposited hydrated amorphous calcium carbonate, which then quickly dehydrated, and subsequently crystallized into aragonite.
"Amazing chemistry happens at the surface of forming nacre," says Gilbert, observing that calcium atoms are involved in the change of amorphous calcium carbonate into crystalline aragonite. At first, the calcium atoms are bonded to six oxygen atoms, and finally to nine of them in the crystalline biomineral.
"It is how the atoms are arranged that matters. The actual chemical composition of calcium carbonate does not change. Only the structure does upon crystallization." This was a huge surprise to the researchers, and Gilbert: "The change in atomic symmetry around calcium atoms, from six to nine oxygen atoms, surprised us. Everyone expected to find amorphous precursor minerals that already had the symmetry of the final crystal at the atomic scale, lacking only the long-range order of the crystals. We stand corrected."
Gilbert states that this fresh insight into the way nature forms mother-of-pearl may soon be applied in industrial applications, such as for the creation of very durable bone implants with this environmentally friendly material.
The research received support from the National Science Foundation and the Department of Energy.