May 17 2019
A novel bio-inspired material could provide a cost-effective and eco-friendly method for recovering uranium from seawater, as recently demonstrated by researchers.
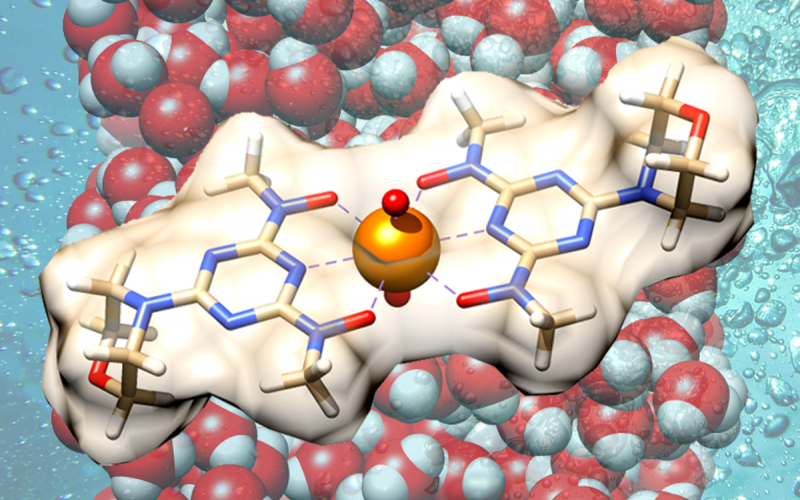 Combining fundamental chemistry with high-performance computing resources at ORNL, researchers demonstrate a more efficient method for recovering uranium from seawater, unveiling a prototype material that outperforms best-in-class uranium adsorbents. (Image credit: Alexander Ivanov/Oak Ridge National Laboratory, U.S. Dept. of Energy.)
Combining fundamental chemistry with high-performance computing resources at ORNL, researchers demonstrate a more efficient method for recovering uranium from seawater, unveiling a prototype material that outperforms best-in-class uranium adsorbents. (Image credit: Alexander Ivanov/Oak Ridge National Laboratory, U.S. Dept. of Energy.)
The unique material, developed by a team of researchers from the Department of Energy’s Oak Ridge and Lawrence Berkeley National Laboratories, the University of South Florida, and the University of California, Berkeley, selectively binds dissolved uranium using a cost-effective polymer adsorbent.
Reported in Nature Communications, the results could help overcome bottlenecks associated with the efficiency and cost of extracting uranium resources from seas and oceans for producing energy in a sustainable manner.
Our approach is a significant leap forward. Our material is tailor-made for selecting uranium over other metals present in seawater and can easily be recycled for reuse, making it much more practical and efficient than previously developed adsorbents.
Ilja Popovs, Study Coauthor¸ Chemical Sciences Division, ORNL
The chemistry of iron-hungry microbes inspired Popovs. Microorganism like fungi and bacteria secrete natural compounds called “siderophores” to drain off key nutrients like iron from their hosts. “We essentially created an artificial siderophore to improve the way materials select and bind uranium,” Popovs stated.
Experimental and computational techniques were used by the team to develop a new functional group called “H2BHT”—2,6-bis[hydroxy(methyl)amino]-4-morpholino-1,3,5-triazine—that specifically chooses water-soluble uranium or uranyl ions over contending metal ions from other elements present in seawater, for example, vanadium.
This major finding is backed by the encouraging performance of a proof-of-principle H2BHT polymer adsorbent. Moreover, due to the special chemistry of H2BHT, uranyl ions are instantly “adsorbed” or adhered to the surface of the material’s fibers. Among other synthetic materials, the model stands out when it comes to boosting the storage space for uranium, producing a recyclable and highly selective material that is able to recover uranium in a more efficient manner when compared to the earlier techniques.
Through a practical recovery technique, extraction of saltwater provides a sustainable option to land-mining uranium that can possibly sustain the production of nuclear power for millennia.
The natural erosion of ore-containing soil and rocks has made uranium deposits replenishable and abundant in seawater. In spite of dilute concentrations—about 3 mg of uranium for each ton of seawater—the oceans across the world contain huge amounts of uranium with an estimated total of four billion tons, which are 1000 times greater supply when compared to all the land sources combined together.
However, ever since the 1960s, the development of efficient uranium adsorbents to tap this promising resource has remained an elusive quest.
The goal is to develop efficient adsorbent materials at a low cost that can be processed using mild conditions to recover uranium, and also reused for multiple extraction cycles.
Alexander Ivanov, Research Scientist, ORNL
Ivanov carried out the computational studies of H2BHT.
The research team, supported by the DOE Office of Nuclear Energy’s Fuel Cycle Research and Development program, has concentrated on establishing the underlying aspects that affect selectivity and boost the amount of recoverable uranium with innovative materials.
Earlier studies on compounds based on amidoxime showed an essentially stronger affinity to vanadium over uranium that might not be easy to overcome. The H2BHT development provides an alternative method, utilizing non-amidoxime materials, to target the uranium element in mixed-metal water environments in a better way.
Traditionally, selectivity has been a barrier toward realizing more efficient adsorbent materials. Previous developments, fueled by trial and error, discovered that uranium is effectively bound in water by the amidoxime-based functional groups. However, these functional groups also do a much better job of recovering vanadium, even though the concentration of this element in seawater is relatively less than uranium.
The result is that amidoxime-based materials, the current front-runners for commercially available adsorbents, fill up more quickly with vanadium than uranium, which is difficult and costly to remove.
Ilja Popovs, Study Coauthor¸ Chemical Sciences Division, ORNL
The extremely concentrated acidic solutions used for eliminating vanadium are very costly when compared to basic or mild processing solutions and they are also burdened by caustic waste streams. In addition, material fibers can be damaged by acid processing, limiting their reuse and making their adoption at the commercial scale extremely costly.
To work as a scaled-up concept, ideally, unwanted elements would not be adsorbed or could easily be stripped during processing and the material reused for several cycles to maximize the amount of uranium collected.
Ilja Popovs, Study Coauthor¸ Chemical Sciences Division, ORNL
The H2BHT polymer is different from vanadium-laden materials and can be processed using mild basic solutions and recycled for prolonged reuse. The features are eco-friendly and also provide substantial cost benefits to promising real-world applications.
The subsequent step is to further refine the method for better efficiency as well as commercial-scale opportunities, the researchers stated. The journal article has been published as “Siderophore-Inspired Chelator Hijacks Uranium from Aqueous Medium.”
The study was supported by DOE’s Office of Nuclear Energy.