Mar 23 2021
In the path toward mitigating the energy crisis and realizing the goal of carbon neutrality, one of the potential methods is the hydrogenation of carbon dioxide (CO2) into methanol by using a “green hydrogen” source based on renewable energy.
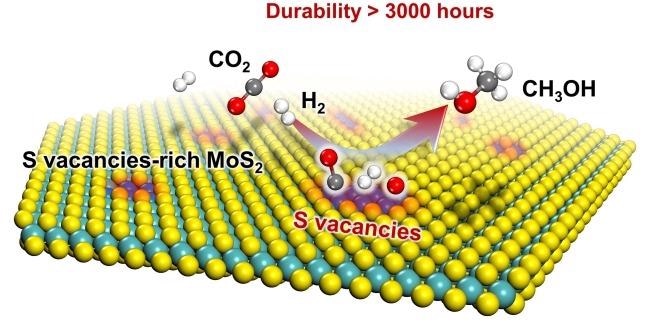 Sulfur vacancies-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Image Credit: Jingting Hu and Liang Yu.
Sulfur vacancies-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Image Credit: Jingting Hu and Liang Yu.
For the first time, a team of researchers headed by Prof. Dehui Deng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), together with Prof. Ye Wang from the College of Chemistry and Chemical Engineering, Xiamen University, recently hydrogenated CO2 into methanol with high efficiency and at low temperature.
The findings of the research were published on March 22nd, 2021, in the Nature Catalysis journal.
Conventional metal oxide catalysts for CO2 to methanol reaction often necessitate a high temperature of more than 300 ℃, which leads to unwanted reverse water-gas shift (RWGS) side reactions, thereby generating huge amounts of CO as the by-product.
The activation of H2 can be promoted by introducing transition metal components onto metal oxides, thus bringing down the reaction temperature. However, this technique also results in excessive hydrogenation of CO2 to CH4, causing lower methanol selectivity.
The sulfur vacancy-rich few-layered MoS2 used as the catalyst in this study could activate and dissociate CO2 and H2 at the same time, at low temperatures and even at ambient temperature. This enabled the hydrogenation of CO2 to methanol with high selectivity and activity.
In situ characterizations together with theoretical calculations showed the in-plane sulfur vacancies on MoS2 as the active spots for the catalysis of the highly selective hydrogenation of CO2 to methanol.
The team discovered that this technique effectively prevented the RWGS reaction and excessive hydrogenation of methanol to CH4. At a temperature of 180 ℃, the selectivity of methanol could reach 94.3% at a 12.5% CO2 conversion.
This result was better than what was achieved by using the commercial Cu/ZnO/Al2O3 catalyst and other catalysts reported earlier.
The selectivity and activity were maintained steadily for more than 3000 hours over the MoS2 catalyst, which makes it a potential candidate for industrial applications.
This work reveals the potential of in-plane vacancies in two-dimensional materials for catalysis and provides a novel strategy for the development of new catalysts to be used in CO2 hydrogenation.
Dehui Deng, Professor, Dalian Institute of Chemical Physics, Chinese Academy of Sciences
This study was highlighted in a News & Views article in Nature Catalysis. Prof. Felix Studt from Karlsruhe Institute of Technology rated it as a phenomenal and intriguing study, which might help achieve large efficiency gains in the conversion of CO2 to methanol.
Journal Reference:
Hu, J., et al. (2021) Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nature Catalysis. doi.org/10.1038/s41929-021-00584-3.