|
The continuing trend toward higher circuit density in microelectronic devices has motivated research efforts in varieties of high-resolution lithography techniques, including electron beam (EB), X-ray, and deep UV irradiation. Use of ultra-thin films and new materials have been proposed as approaches to improve resolution in lithography. The Langmuir-Blodgett (LB) technique is very effective method used to prepare well-defined ultra-thin film with controlled thickness and orientation at a molecular level. Therefore, LB films are expected to realize ultra-high resolution photolithography [1-4].
In previous studies, [5-7] we have found that N-octadecylacrylamide forms a uniform LB film with a highly ordered structure, and yielded a fine negative pattern by photopolymerization. Furthermore, we have also succeeded in the preparation of preformed polymer LB film that has a cross-linking group [8]. By the cross-linking reaction with deep UV and electron beam irradiation we obtained a fine negative pattern consisting of two-dimensional network. All of these polymer LB films resulted in negative-tone photopatterns. On the other hand, we also obtained positive type photopatterns using poly(N-tetradecylmethacrylamide)(p(TDMA)) LB films without any development process (self-development) [9, 10]. It was found that the higher sensitivity could be obtained by changing the alkyl side chain to the short-branched type [11]. In addition, the deprotection reaction of t-butoxycarbonyloxy group has also been used in positive patterning of polymer LB films [12-14]. Combining these interesting properties, the improvement of not only the sensitivity but also the imaging quality can be expected. In this work, we prepared the copolymers of photodegradable N-tetradecylmethacrylamide (TDMA) with t-butyl 4-vinylphenyl carbonate (tBVPC) (Figure 1) aiming at the fabrication of a new type of positive resist taking place both main chain scission and polarity change caused by t-butoxycarbonyloxy group deprotection.
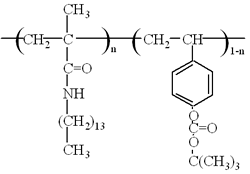
Figure 1. Chemical structure of p(TDMA-tBVPC).
Experimental
The copolymer (p(TDMA-tBVPC)) was prepared by free-radical copolymerization of N-tetradcylmethacrylamide (TDMA) with t-butyl 4-vinylphenyl carbonate (tBVPC) in toluene at 60˚C. Measurement of surface pressure ( π) - area (A) isotherm and deposition of monolayers were carried out at 15˚C with a Langmuir trough system (FSD-50 and 51, USI) with a compression speed of 14 cm2/min. The rate of deposition was set at 10 mm/min at both up- and down-strokes. Deionized pure water (Milli-QII, MILLIPORE) was used as the subphase. The copolymer was dissolved in chloroform at a concentration of ca. 1 mM and the solution was spread on the water surface. The glass, quartz, and silicon slides on which LB film was deposited were initially cleaned by a UV-O3 cleaner (NL-UV253, Nippon Laser Electronic); then they were made hydrophobic with n-octyltrichlorosilane. UV absorption measurements were recorded with a Hitachi U-3000 UV-Vis spectrophotometer. The molar ratio of the tBCPV in the copolymer were determined from 1H NMR of p(TDMA-tBVPC). Molecular weight was determined with a Toyo Soda gel permeation chromatography (GPC) using a polystyrene standard. IR spectra were measured with a JASCO-IR 230 spectrometer. Deep UV irradiation was carried out with a deep UV lamp (UXM-501MA, USHIO) through an IR-cut filter. The thickness of copolymer LB film was determined with surface profilometry using a Sloan Dektak 3ST. Gold film was deposited onto a glass substrate with a vacuum evaporator (V-KS200, Osaka Vacuum, Ltd).
Results and Discussion
Formation of Copolymer LB Films
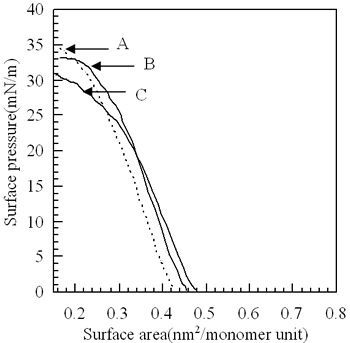
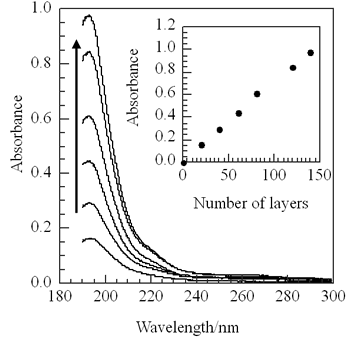
The molecular weight of p(TDMA-tBVPC) are summarized in Table 1. The copolymer(p(TDMA-tBVPC)) was spread on the water subphase from chloroform solution (ca. 1mM) to measure surface pressure (π) - area(A) isotherms (Figure 2). The copolymer p(TDMA-tBVPC) monomers have collapse pressure. Their curves stand sharply. We can conclude that they can form a condensed monomer on the water subphase. The p(TDMA-tBVPC) monolayer could be transferred onto a solid substrate with a transfer ratio of almost unity. UV absorption spectra of p(TDMA-tBVPC56) LB film on quartz were measured as a function of the number of layers (Figure 3). The absorbance at 193 nm apparently increases linearly with the number of layers deposited, indicating the regular deposition of the copolymer monolayer onto the solid substrate.
Table 1. Various copolymers of TDMA with tBVPC.
|
|
|
p(DDMA-tBVPC23)
|
23
|
0.96
|
2.20
|
2.29
|
|
p(DDMA-tBVPC35)
|
35
|
1.21
|
2.10
|
1.73
|
|
p(DDMA-tBVPC56)
|
53
|
2.60
|
4.10
|
1.58
|
|
|
|
Figure 2. Surface pressure(π) –(A) area isotherms of p(TDMA-tBVPC) measured at 15˚C. (A: p(TDMA-tBVPC23), B: p(TDMA-tBVPC35), C: p(TDMA-tBVPC56).
|
|
|
|
Figure 3. UV absorption spectra of p(TDMA-tBVPC65) LB films as a function of deposited layers. Inset; plots of the absorbance at 193 nm vs. the number of LB films deposited.
|
Photolithographic Properties of p(TDMA-tBVPC) LB Films
The p(TDMA-tBVPC56) LB film with 60 layers was directly exposed to a deep UV lamp (86mW/cm2) through a photomask without no monochromatic filter in the air for 60 min and then developed with 1% tetramethylammonium hydroxide (TMAH) aqueous solution. As shown in Figure 4, we obtained a clear positive-tone pattern with a resolution of 0.75 µm, which is the resolution limit of the photomask employed in the present work. Among the photopatterns of the LB films (20, 40, 60, and 80 layers), the LB film with 60 layers gave the finest resolution under the present condition. Therefore, the subsequent experiments for the sensitivity were carried out with 60 layers of LB films.
Figure 4. An optical micrograph of p(TDMA-tBVPC56) LV film with 60 layers irradicated with deep UV lamp for 60 mins in air followed by the development with 1% TMAH aqueous solution for 20s
To estimate the sensitivity of lithographic property for the LB film, the residual thickness of the LB films in the exposed portion was measured as a function of the exposure time (Figure 5). It should be noted that the p(TDMA-tBVPC56) LB films are more sensitive than the homopolymer [p(TDMA)] LB film (Figure 5) [11]. Moreover, there are apparently two steps in the decrease in the thickness of the p(TDMA-tBVPC56) LB film, while the monotonic decrease in the thickness was observed in the sensitivity curve of all poly(alkylmethacrylamide) LB films which undergo main chain scission [11]. Since the light intensity at 193 nm (λmax of the copolymers) from the deep UV lamp was very weak and was not able to be estimated exactly, the quantitative analysis of the sensitivity of the LB films could not be determined. From the shape of the sensitivity curve of p(TDMA-tBVPC56)LB films, however, it is noticeable that there are two steps in the process of photodecomposion; the deprotection of t-butoxycarbonyloxy group is predominant in the initial region and consequently the main chain scission due to the TDMA fragmentation occurs. The normalized film thickness decreases with exposure time, that is, the copolymer in the LB film is effectively decomposed by deep UV irradiation and removed completely with development of alkaline solution.
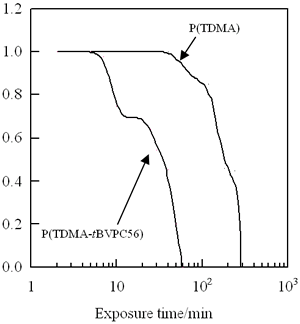
Figure 5. Sensitivity curves of p(TDMA-tBVPC56) and p(TDMA) LB films.
Etching Resistance
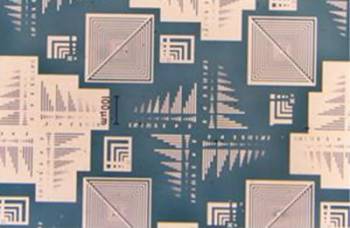
Etching of p(TDMA-tBVPC56) LB film against wet etching was also investigated as follows: the p(TDMA-tBVPC56) LB film with 20 layers was deposited on a gold substrate; then, it was irradiated through a photomask with deep UV lamp (86 nW/cm2); finally, it was developed with alkaline solution. Next, the positive-tone patterned substrate was immersed into a mixed solution of ammonium iodide, iodide, ethanol and water (etchant). Finally the LB film on gold film was removed with chloroform. Figure 6 shows the patterns of gold film after the etching. Resolution of 1.0 µm for gold film was obtained. This indicates that the copolymer p(TDMA-tBVPC56) LB film with at least 20 layers has a high etching resistance for etchant.
|
|
|
Figure 6. Etched pattern of gold film on the glass substrate.
|
Photodecomposition of Copolymer LB Films
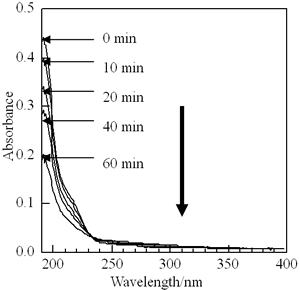
The photopattern formation in copolymer LB films was investigated for the photodecomposition mechanism. To obtain the significant change in the experimental data, p(TDMA-tBVPC56) LB film with 40 layers were used for UV and IR spectra, and p(TDMA-tBVPC56) cast film was utilized for GPC measurements. First, absorption spectra change of p(TDMA-tBVPC56) LB film with 60 layers under deep UV irradiation was measured (Figure 7). The absorption band at 193 nm is assigned to the amide group and the group of t-butoxycarbonyloxy group. The decrease in the absorbance at 193 nm indicates that the main chain scission, photodecomposition of aromatic ring or t-butoxycarbonyloxy group occurs by UV light irradiation, leading to the formation of volatile low molecular weight compounds such as carbon dioxide, isobutylene [16], or form conjugated structure because of the presence of a broad absorptive tail at wavelength above 230 nm.
|
|
|
Figure 7. Changes of UV absorption spectra of p(TDMA-tBVPC56) LB films with 70 layers by deep UV irradiation at ambient air.
|
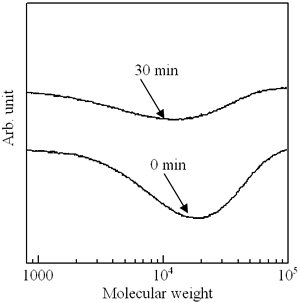
In addition, the GPC measurement of the p(TDMA-tBVPC56) cast film was carried out to confirm the molecular weight change during the deep UV irradiation. As clearly shown in Figure 8, the molecular weight decreased with irradiation time, and the curve became broader, indicating that the molecular distribution is larger than that of the initial state. These findings suggest that the main chain scission also takes place.
|
|
|
Figure 8. GPC curves of p(TDMA-tBVPC56) cast film as a function of exposure time of deep UV irradiation.
|
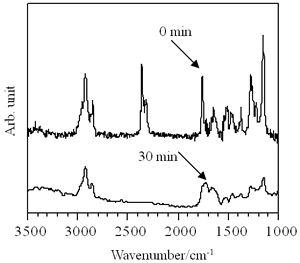
The absorbance at 3350 cm-1 in the IR spectra of the LB film, assigned to the stretching vibration of hydroxy group, was increased after irradiation (Figure 9), which is the evidence that hydroxy group was produced by UV irradiation [11, 17]. The band at 1750 cm-1, which is assigned to carbonyl group, is attenuated by deep UV irradiation. This also means that the t-butoxycarbonyloxy group is removed from the side chain to give a phenol group which can be dissolved in alkaline solution [15]. Moreover, the absorbance at 2910 cm-1 assigned to the alkyl group is drastically decreased after 30 min irradiation. This implies that the LB film is more dissolved in alkaline solution due to the change of the molecular weight. Although the difficulty still remains in distinguishing the difference between main chain and side chain scissions, these findings support the above discussion in the sensitivity curve for p(TDMA-tBVPC56) LB film.
|
|
|
Figure 9. IR spectra of p(TDMA-tBVPC56) LB films with 80 layers by deep UV irradiation.
|
Conclusions
The copolymers [p(TDMA-tBVPC)] were prepared by free radical copolymerization. The copolymer has a structure being subjected to decomposition in main chain scission and side chain cleavage. The polymer forms a stable monolayer on the water subphase and the monolayer can be transferred onto a solid substrate. On irradiation with deep UV light on the LB film, the positive tone patterns could be obtained after development with an alkaline aqueous solution. We also investigated the etching property of the underling gold. The p(TDMA-tBVPC56) LB films with 20 layers have a high etching resistance with a resolution of 1.0 µm. From the results we expect that the new copolymer [p(TDMA-tBVPC56)] can be used in the lithographic process in future.
Acknowledgements
We would like to thank Prof. T. Miyazaki, and Dr. Y. Ando, Department of Applied Physics, Graduate School Engineering, Tohoku University, for the use of the surface profilometry. This work was partially supported by Grant-in-Aid for the “Research for the Future” Program (JSPS-RFTF97P00302) from the Japan Society for the Promotion of Science.
References
- K. B. Blodgett, “Films Built by Depositing Successive Monomolecular Layers on a Solid Surface” J.Am. Chem. Soc., 57 (1935) 1007.
- K. B. Blodgett and I. Langmuir, “Built-Up Films of Barium Stearate and Their Optical Properties”, Phys. Rev., 51 (1937) 964.
- S. W. J. Kuan, C. W. Frank, C. C. Fu, D. R. Allee, P. Maccagno and R. F. W. Pease, “Ultrathin polymer films for microlithography”, J. Vac. Sci. Technol., B6 (1988) 2227.
- T. Yoshimura and N. Asai, Jpn. J. Appl. Phys., 33, L970 (1994).
- T. Miyashita, Y. Mizuta and M. Matsuda, “Studies on Langmuir-Blodgett Multilayer Formation from Preformed Poly(N-alkylacrylamides)”, Br. Polym. J., 22 (1990) 327.
- T. Miyashita, H. Yoshida and M. Matsuda, “Drawing Fine Patterns on N-Octadecylacrylamide Langmuir-Blodgett Multilayers: A New Class of Ultrathin Resists”, Thin Solid Films, 155, L11 (1987).
- X. D. Li, A. Aoki and T. Miyashita, “Formation of Polymerizable Monomer Langmuir-Blodgett Films with Polyfluorocarbon Chains for Use in High-Resolution Negative Resists”, Macromolecules, 30 (1997) 2194.
- A. Aoki, M. Nakaya and T. Miyashita, “Drawing Fine Patterns with Cross-Linkable Polymer Langmuir-Blodgett Film”, Chem. Lett., (1996), 667.
- Y. Guo, F. Feng and T Miyashita, “A New Type of Dry-developed Positive Deep UV Resist Using Polymer Langmuir-Blodgett Films”, Chem. Lett., (1998) 1269.
- Y. Guo, F. Feng and T. Miyashita, “Preparation of Poly(N-alkylmethacrylamide) Langmuir-Blodgett Films for the Application to a Novel Dry-Developed Positive Deep UV Resist”, Macromolecules, 32 (1999) 1115.
- Y. Guo, M. Mitsuishi and T. Miyashita, “Poly(N-alkylmethacrylamide) LB Films with Short-Branched Alkyl Side Chains for a Self-Developed Positive Photoresist”, Macromolecules, 34 (2001) 3548.
- T. Li, M. Mitsuishi and T. Miyashita, “Preparation of a New Photodegradable Copolymer LB Film and Its Application to Photopatterning”, Chem. Lett., (2000) 608.
- T. Li, M. Mitsuishi and T. Miyashita, “Photodegradable polymer LB films for nano-lithographic imaging techniques”, Thin Solid Films, 389 (2001) 267.
- T. Li, M. Mitsuishi and T. Miyashita, “Positive-tone Imaging of Photodecomposable Polymer LB Films”, Bull. Chem. Soc. Jpn., 74 (2001) 1757-1760.
- G. L. Gaines Jr, “Solvatochromic compound as an acid indicator in nonaqueous media”, Anal. Chem., 48 (1976) 450.
- J. M. J. Fréchet, T. G. Tessier, C. G. Willson and H. Ito, “Poly[p-(formyloxy)styrene]: synthesis and radiation-induced decarbonylation”, Macromolecules, 18 (1985) 317.
- M. Mitsuishi, T. Li and T. Miyashita, “In situ Observation of Deep UV Patterning in Polymer LB Films By Surface Plasmon Spectroscopy”, Mol. Cryst. Liq. Cryst., 370 (2001) 269-272.
|