| Product # |
Description |
Sizes |
| 27-0477 |
Cobalt-dppe heterogeneous water oxidation catalyst (~17%-Co) |
100 mg
500 mg |
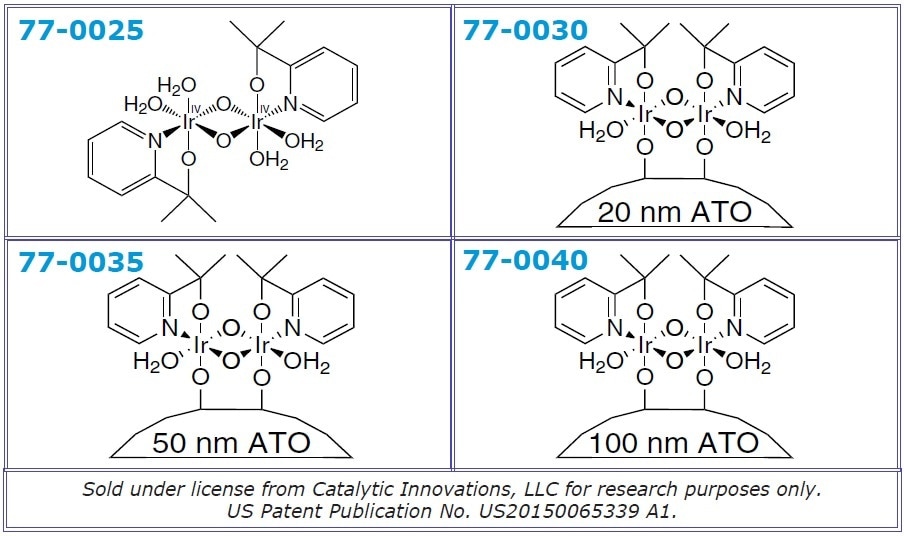
| 77-0025 |
[2-(Pyridine-2-yl)-2-propanato]iridium(IV) dimer solution 97% (1 mM in 0.1 Molar aqueous NaIO3) |
10 ml
50 ml |
| 77-0030 |
Antimony Tin Oxide/Iridium Het-WOC core/shell nanopowder, 20 nm (conductive and acid-stable) |
250 mg
1 g |
| 77-0035 |
Antimony Tin Oxide/Iridium Het-WOC core/shell nanopowder, 50 nm (conductive and acid-stable) |
250 mg
1 g |
| 77-0040 |
Antimony Tin Oxide/Iridium Het-WOC core/shell nanopowder, 100 nm (conductive and acid-stable) |
250 mg
1 g |
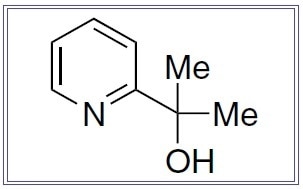
| 07-3333 |
2-(Pyridine-2-yl)propan-2-ol, min. 95% pyalc |
100 mg
500 mg
2 g |
Applications
- OER scaffold in electrolysis
- ORR scaffold in fuel cells
- Corrosion resistance
- Wastewater remediation
- Oxidation catalysis
Ligand also available:
07-3333
Cobalt Catalyst and Nitrogen Ligand
| . |
. |
. |
| 27-0477 |
Cobalt-dppe heterogeneous water oxidation catalyst (~17%-Co)
(C26H24CoP2); light-brown powder. |
100 mg
500 mg |
| 07-3333 |
2-(Pyridine-2-yl)propan-2-ol, min. 95% pyalc
(C8H11NO); F.W. 137.18; white xtl. |
100 mg
500 mg
2 g |
Molecular Iridium Monolayer
| 77-0025 |
2-(pyridine-2-yl)-2-propanato]iridium(IV) dimer solution 97% (1 mM in 0.1 Molar aqueous NaIO3)
(C16H28Ir2N2O8); F.W. 728.84; blue liq. |
10 ml
50 ml |
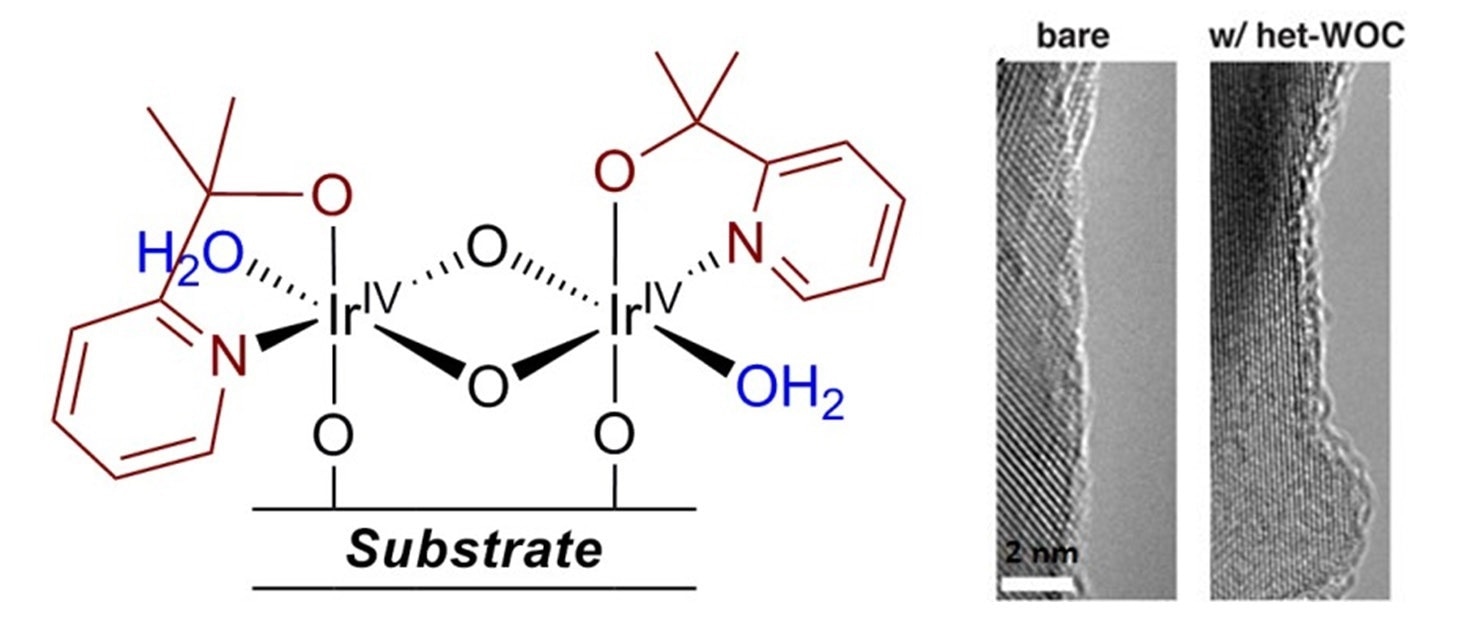
Standard Operating Procedure
Material heterogenization is a simple process and can be carried out in the following conditions: at ambient temperature, in air, with no additives, applied potential or other treatments. For most metal oxide or carbon-based substrates:
- Dip the substrate in het-WOC deposition solution (or disperse powders, if powder).
- Wait 4 to 12 hours (typically overnight).
- Remove the substrate from solution (or filter out powder) and wash with clean water.
This will result in the deposition of the monolayer Ir material on the substrate, with a surface structure as shown1 (TEM image on iron oxide shown to the right):
In order to increase the coverage of het-WOC deposition solution across large substrates, the solution can be diluted.
As mentioned in the SDS, the het-WOC deposition solution mostly contains water, therefore dilution with water is best. Also, the het-WOC deposition solution can be re-used repetitively to load numerous substrates with the Ir monolayer. Each loading employs just a small amount of the Ir present in solution, albeit this depends on the substrate’s surface area.
Acid Stable Iridium-Decorated Conductive Oxides
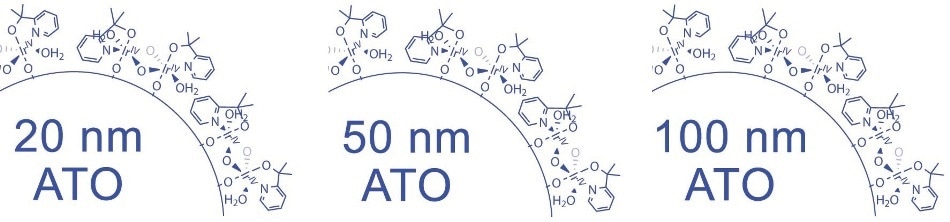
| Product Number |
Product Description |
Particle Size |
BET Surface area |
Resisitivity |
| 77-0030 |
Antimony Tin Oxide/Iridium Het-WOC core/shell nanopowder, 20 nm (conductive and acid-stable) |
20 nm |
50 - 60 m2/g |
0.3 - 0.7 Ω•cm |
| 77-0035 |
Antimony Tin Oxide/Iridium Het-WOC core/shell nanopowder, 50 nm (conductive and acid-stable) |
50 nm |
40 - 50 m2/g |
0.05 - 0.08 Ω•cm |
| 77-0040 |
Antimony Tin Oxide/Iridium Het-WOC core/shell nanopowder, 100 nm (conductive and acid-stable) |
100 nm |
5 - 10 m2/g |
0.05 - 0.08 Ω•cm |
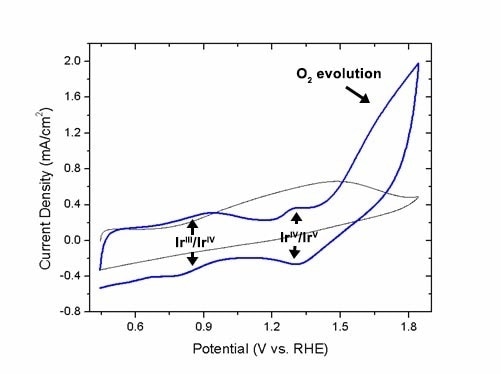
Cyclic voltammogram of 50 nm ATO/het-WOC core/shell nanopowder operating in a test electrolyzer (built-in Hg/HgSO4 reference) for oxygen evolution (blue) compared to a bare 50 nm ATO control (grey). Redox features of the molecular iridium species are present, as well as the catalytic wave for oxygen evolution. Activity persists for over 90 days.
Find Out More About Complexes for Catalytic Water Oxidation
References and Further Reading
- J. Am. Chem. Soc., 2013, 135, 10837.
- J. Am. Chem. Soc., 2014, 136, 13826.
- Nat. Commun., 2015, 6, 6469.
- Angew. Chem. Int. Ed., 2015, 54, 11428.
- Energy Environ. Sci., 2016, 9, 1794.

This information has been sourced, reviewed and adapted from materials provided by Strem Chemicals.
For more information on this source, please visit Strem Chemicals.