Selected ion flow tube mass spectrometry (SIFT-MS) allows fast and sensitive measurement of formaldehyde in fragrance samples using headspace analysis. Matrix effects due to the variable headspace compositions of the fragrance mixes were successfully dealt with by applying the method of standard additions.

Image Credit: Pixel-Shot/Shutterstock.com
This study analyzed formaldehyde content in three aqueous fragrances with results ranging from 7 to 85 μg mL-1.
The high sample throughput of SIFT-MS ensures that the calibration curves for each matrix are produced quickly, making the standard additions method more cost-effective when using SIFT-MS compared to chromatographic methods.
This allows SIFT-MS to decrease the time needed to report the initial results while enhancing the daily throughput.
For full standard additions on each sample, three complete analyses (including standards) can be realized per hour. If the matrix is uniform, 12 samples can be screened per hour.
The simplicity with which SIFT-MS quickly examines formaldehyde makes it well-suited for product quality control – both in testing laboratories and on the process line.
Introduction
Volatile compounds can be dangerous for human health if present in consumer or medicinal products at levels that represent a toxicological risk.
Formaldehyde is of specific concern since it is a well-known human carcinogen and can emerge from impurities in, or degradation of, ingredients.1,10 It can also arise from lingering impurities in packaging materials.
Formaldehyde, however, is challenging to analyze using traditional chromatographic methods (such as gas and liquid chromatography, GC, and LC) because of its high polarity, increased reactivity, and the need for sample preconcentration and solvent extraction.
Selected ion flow tube mass spectrometry (SIFT-MS) directly analyzes formaldehyde in headspace at toxicologically significant concentrations. This article employs SIFT-MS to analyze formaldehyde in three fragrance mixes.
These mixes exhibit various compositions, but they all challenge conventional SIFT-MS. This is because the total matrix volatile organic compounds (VOCs) concentration surpasses the instrument’s dynamic range.2,8
This difficulty emerges because of the direct, chromatography-free analysis performed using SIFT-MS. This means that analytes and other matrix volatiles pass through the instrument ionization region concurrently. This issue can be solved by applying the method of standard additions to SIFT-MS.5,7
This approach, combined with chromatographic techniques when examining complex matrices, allows the SIFT-MS instrument to measure trace components even when the instrument is working above its traditional dynamic range.
As shown below, formaldehyde is effectively measured in the fragrance matrix by using this method. Moreover, the application of automated headspace-SIFT-MS brings substantial workflow benefits compared to using standard additions with chromatographic approaches.
These benefits include reporting results more than 1.8-fold quicker and delivering over 2.9-fold higher sample throughput. This shows that standard additions are a practical and cost-effective analytical method in the extensive SIFT-MS analysis toolkit.
Method
1. The SIFT-MS Technique
This study used a Syft TracerTM SIFT-MS instrument working on helium carrier gas. SIFT-MS (Figure 1) employs soft chemical ionization (CI) to produce mass-selected reagent ions that can quickly react with and measure VOCs as low as part-per-trillion concentrations (by volume, pptV).8
Syft TracerTM instruments offer up to eight reagent ions (H3O+, NO+, O2+, O-, OH-, O2-, NO2-, and NO3-) generated through a microwave discharge in air. These reagent ions react with VOCs and other trace analytes in well-controlled ion-molecule reactions. However, they do not react with the main constituents of air (N2, O2, and Ar).
This allows for direct and real-time analysis of air samples at trace and ultra-trace levels without the need for pre-concentration. Quick substitution between reagent ions offers enhanced selectivity as multiple reaction mechanisms provide independent measurements for each analyte.2
The multiple reagent ions eliminate uncertainty from isobaric overlaps in mixtures comprising multiple analytes.
Syft TracerTM, therefore, established the benchmark for selective and sensitive real-time analysis of volatile compounds. The automated analysis used a Syft TracerTM with a multipurpose autosampler (MPS Robotic Pro, GERSTEL; Mülheim, Germany).
[Note: The best configuration is a dual-head GERSTEL MPS Robotic Pro, while the next best option is the single-head GERSTEL MPS Robotic Pro with tool change capability.]
The autosampler was controlled with the help of GERSTEL’s Maestro software, and incubation took place in a GERSTEL agitator.
Headspace was sampled using a 2.5-mL headspace syringe (heated to 150 °C) and then injected at a flow rate of 100 μL s-1 into the autosampler inlet of the SIFT-MS instrument (heated to 150 °C) through a self-sealing GERSTEL septum-less sampling head.
Because the nominal sample flow into the SIFT-MS instrument is 420 µL s-1, the sampling head also introduced a make-up gas flow (consisting of ultra-high purity nitrogen). Figure 2 demonstrates the simultaneous sample injection and examination of formaldehyde for the sample and three standard additions.
The analysis time for each sample was 100 seconds and reported concentrations were derived from the mean of measurements made during injection (between approximately 40 and 60 seconds in Figure 2).
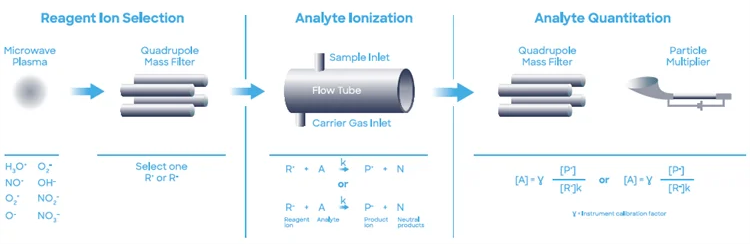
Figure 1. Schematic diagram of the SIFT-MS technique, which utilizes soft chemical-ionization for direct analysis of samples. Image Credit: Syft Technologies
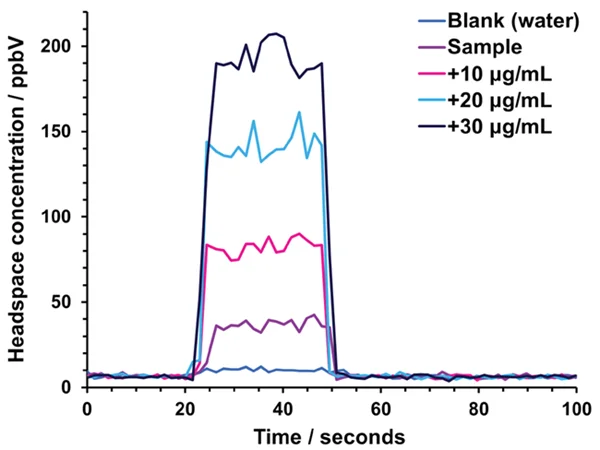
Figure 2. Real-time SIFT-MS analysis of formaldehyde: the headspace injections shown are from Sample 1 and show the measurement made on the sample itself and the three standard additions. Data for a blank measurement are shown for comparison. Image Credit: Syft Technologies
2. SIFT-MS Analysis of Formaldehyde
SIFT-MS selectively identifies formaldehyde through the proton-transfer reaction displayed in Eqn. 1.9
H3CO+ + H2CO → H3CO+ + H2O k=3x10-9 cm3s-1
The H3CO+ product ion is identified at a mass-to-charge ratio (m/z) of 31. This product ion m/z is unique to the detection of formaldehyde because of the soft ionization in SIFT-MS and its rare occurrence with other volatile compounds.
Formaldehyde measurement was carried out using the literature reaction rate coefficient (k) mentioned above. It is important to note that no internal standard was used.
3. Samples
A customer provided three anonymized samples for assessing formaldehyde impurity concentrations.
The samples were aqueous but included substantial concentrations of proprietary fragrance components and a solvent carrier. For 1 mL of fragrance mix was sampled into 20-mL headspace vials. Samples were incubated for 20 minutes at 75 °C.
4. Standard Additions Utilized with SIFT-MS
To minimize the introduction of matrix effects, the volume of the spiked standard was kept as small as possible (10 μL). During method development, it was established that headspace partitioning remained unaffected even after multiple cycles of standard spikes and analyses.
[Note: If headspace partitioning is disturbed, it is recommended that multiple vials per sample should be prepared – one for each spike level.] Therefore, the procedure for each test sample was:
- Incubate
- Inject headspace into the SIFT-MS instrument
- Add spike
This process was repeated for the number of standard additions performed (three or four). The standard additions were introduced at 10, 20, and 30 μg mL-1 from a 1000-μg mL-1 standard. For a method utilizing three standard additions (here, water and samples 1 and 3), the sequence for one sample is depicted in Figure 3(a).
Since the analysis is not the rate-limiting step in SIFT-MS examination (as it is often in the case of chromatographic analysis), running three additional samples concurrently only increases the overall analysis time by 16 minutes (or 17%; Figure 3(b)).
It is important to note, however, that this method does not use the analytical instrument as effectively as preparing individual samples at various spike levels, since this method reverts to static headspace throughputs (as discussed in the next section).
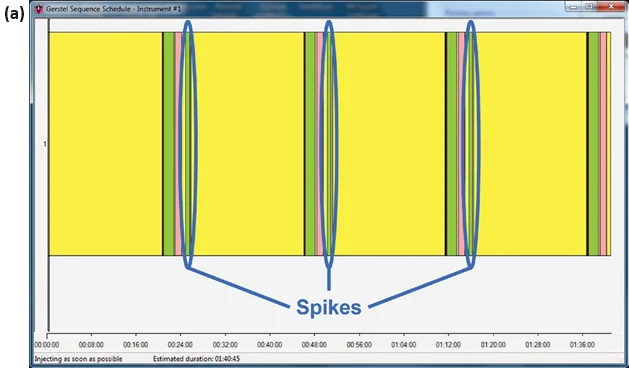
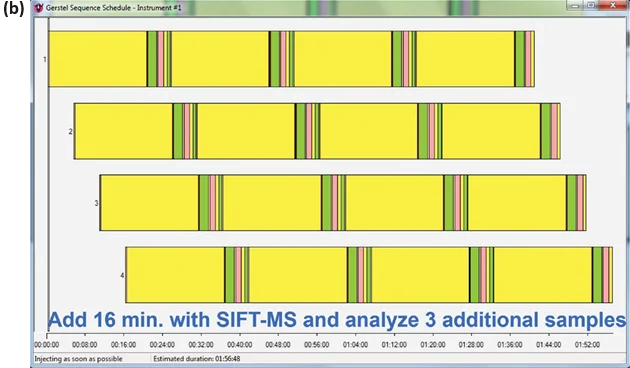
Figure 3. Sequence schedules from the GERSTEL Maestro software package, illustrating the sequences for (a) one and (b) four samples being analyzed using the method of standard additions on an automated SIFT-MS instrument. Image Credit: Syft Technologies
Results and Discussion
The fragrance matrices of variable composition, combined with total VOC concentrations that surpassed the instrument’s linear range, posed a challenge for the reliable analysis of formaldehyde using static headspace SIFT-MS analysis. Two aspects were predominantly important:
- The partitioning of formaldehyde from aqueous solutions was relatively low – leading to comparatively poor sensitivity (a limit of quantitation (LOQ) of about 0.1 μg mL-1)
- High levels of VOCs in the matrix consumed reagent ion signals but were inconsistent from one sample to the other, resulting in an overestimation of concentration.
A straightforward aqueous calibration set for formaldehyde measurement could not be utilized, leading to the adoption of the method of standard additions.
The method was developed on sample 2 using four additions (at 10, 20, 30, and 40 μg mL-1), as depicted in Figure 4(c). However, three additions from 10 – 30 μg mL-1 proved sufficient for this approach (Figure 4(a),(b),(d)).
Figure 4 demonstrates the linearity across the range of standard additions in (a) water and (b) to (d) for the fragrance samples 1, 2, and 3, respectively. Extrapolating the standard addition curves revealed formaldehyde concentrations of 65 μg L-1, 87 μg L-1, and 7 μg L-1 in the respective fragrance samples.
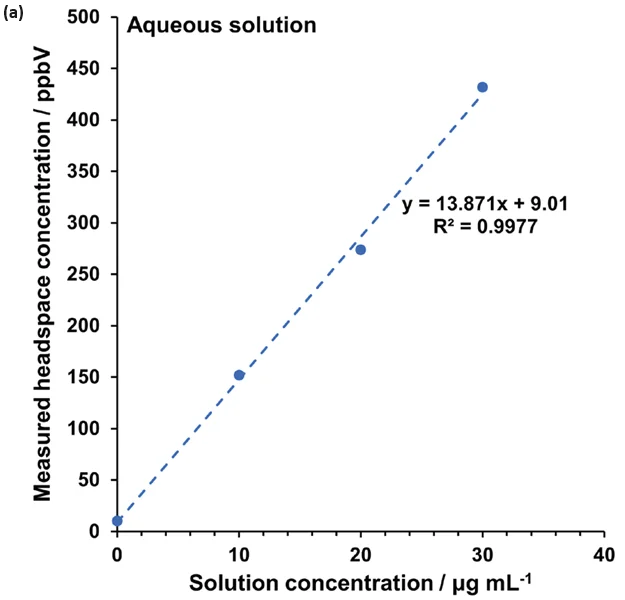
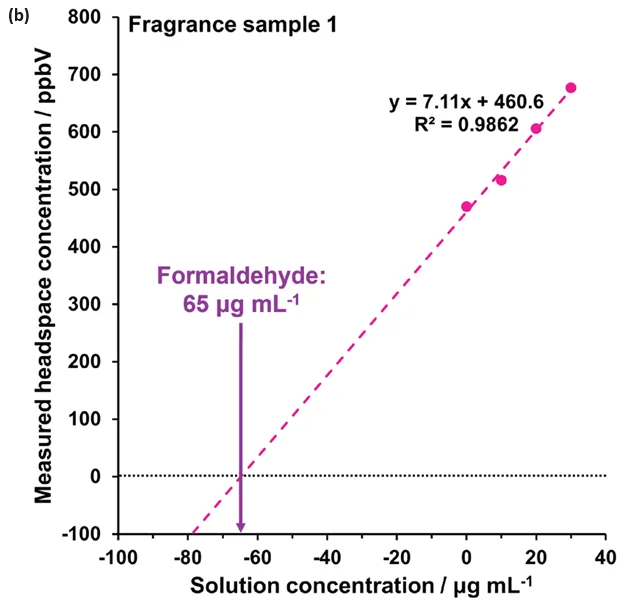
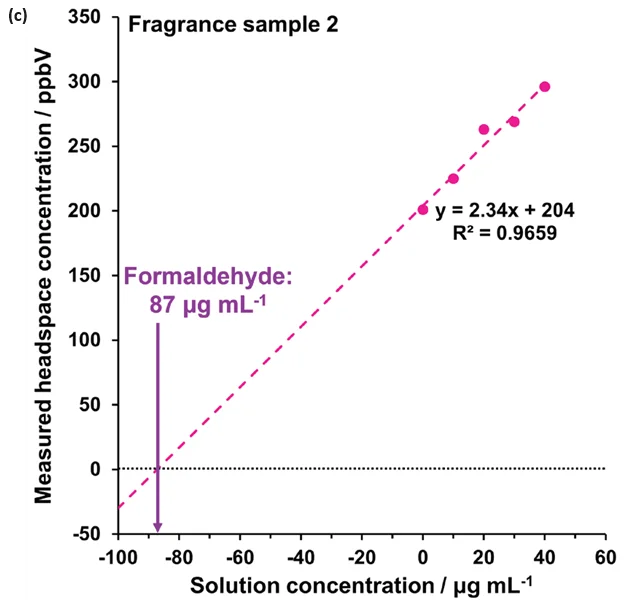
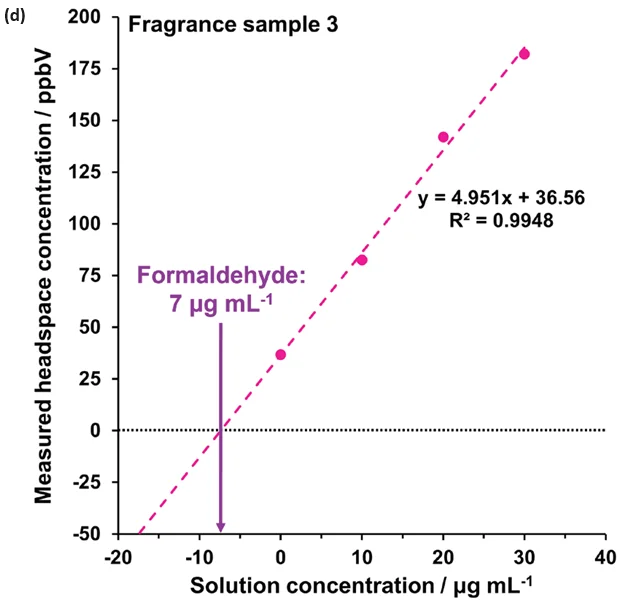
Figure 4. Standard additions calibration curves determined using automated headspace-SIFT-MS for (a) water and (b) – (d) fragrance samples 1 to 3, respectively. Image Credit: Syft Technologies
These findings show that the method of standard additions can be easily applied to SIFT-MS for systems where static headspace analysis poses challenges, but where homogeneous spiked solutions can be prepared.
In cases where this condition cannot be met, multiple headspace extraction (MHE) may be necessary.6
Although the sample preparation method used here is conceptually elegant, it employs just one vial per sample and the standard additions are spiked sequentially. Therefore, it is not the most efficient method.
For SIFT-MS, it takes slightly over 100 minutes to produce a result for the first sample (with 20-minute incubation period) and analyzes approximately 48 samples per day (Figure 3).
For a GC- or LC-based method, based on a run-time of 15 minutes, less than 10 samples per day can be examined because samples cannot be run in parallel, unlike SIFTMS with its shorter run times (Figure 3b).
A more efficient method to running standard additions on both traditional and SIFT-MS platforms involves making individual samples for each spike level up-front using the autosampler, then conducting the sequence as if it were traditional static headspace analysis.
This method is shown in Figure 5(a), assuming that every sample needs full standard additions and disregarding any additional time taken for GC or LC sample preparation.
GC benefits most in terms of daily throughput (still only about one-third of the throughput of SIFT-MS), while the time to report the first result is decreased by 30% for SIFT-MS (to 70 minutes, including preparation time).
If the analysis is repeatedly performed on the same matrix, both techniques allow for subsequent analyses from a single static headspace analysis every 15 minutes for GC and LC, and every 5 minutes for SIFT-MS.
Figure 5(b) displays this technique, incorporating triplicate calibration on three samples to enhance the reliability of the standard additions curve that is applied to the subsequent samples.
The flexibility of the Syft TracerTM platform is equally applicable to routine static headspace analysis and the method of standard additions.
This means that the analyses using standard additions can be seamlessly integrated into a routine analysis workflow with no need for changes in instrument configuration.4
When the same matrix is tested repeatedly, Syft TracerTM can offer high-throughput screening over lengthy periods without the necessity for frequent recalibration, further enhancing its application to quality control (QC) applications.3
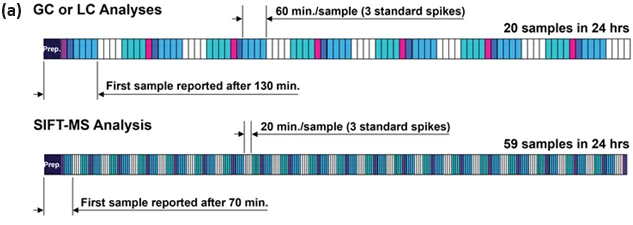
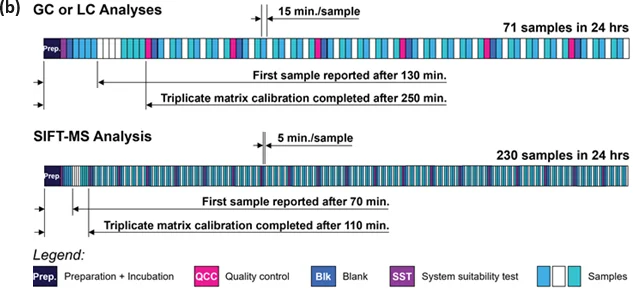
Figure 5. Efficient 24-hour chromatographic and SIFT-MS sequence schedules for analysis of formaldehyde (with 20 min. incubation) using (a) the full method of standard additions on separate spiked samples for every sample tested, and (b) triplicate calibration using standard additions followed by headspace analysis. In both scenarios, three calibration spikes are assumed and these plus the sample are indicated back-to-back using the same fill color. Image Credit: Syft Technologies
Conclusions
- Using ultra-soft chemical ionization allows for the direct analysis of formaldehyde without the need for derivatization or other sample preparation or preconcentration.
- The method of standard additions is well-suited for direct sample analysis in SIFT-MS and enables quantitative analysis of VOCs in more challenging matrices.
- SIFT-MS analyzes formaldehyde with a throughput of 12 samples per hour for static headspace and 3 samples per hour (including standards) for the full method of standard additions.
- The SIFT-MS method offers several productivity benefits, reporting the first result 1.8 times faster than chromatographic techniques and providing a daily throughput increase of 2.9 to 4.8 times.
- Seamless transitions between test methods make Syft TracerTM the most efficient and adaptable instrument for analyzing volatile impurities, particularly in QC applications.
References and Further Reading
- European Commission (2018). Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work, COM/2018/0171 final – 2018/081 (COD).
- Langford VS (2023). SIFT-MS: Quantifying the volatiles you smell… and the toxics you don’t. Chemosensors 11, 111. https://doi.org/10.3390/chemosensors11020111.
- Langford VS, Perkins MJ (2023). Syft TracerTM: Next-generation volatile impurity analysis for enhanced workflows. Syft Technologies application note. Available online: http://bit.ly/3LYH2IP.
- Langford VS, Silva, LP, Perkins MJ (2023). Syft TracerTM: Revolutionary Productivity for Volatile Residue and Impurity Analysis. Syft Technologies application note. Available online: https://bit.ly/3ZU3SEJ.
- Perkins MJ, Langford VS (2021). Application of routine analysis procedures to a direct mass spectrometry technique: Selected ion flow tube mass spectrometry. Rev. Sep. Sci. 3(2), e21003. https://www.researchgate.net/publication/351606309_Application_of_Routine_Analysis_Procedures_to_a_Direct_Mass_Spectrometry_Technique_Selected_Ion_Flow_Tube_Mass_Spectrometry_SIFT-MS.
- Perkins MJ, Langford VS (2022). Multiple Headspace Extraction- [SIFT-MS]. Part 1: A Protocol for Method Development and Transfer to Routine Analysis. Rev Sep Sci. 4(1), e22001. https:// doi.org/10.17145/rss.22.001.
- Silva L, Langford V (2022). High-throughput, quantitative analysis of benzene in personal care products using headspace-SIFT-MS. Syft Technologies application note. Available online: http://bit.ly/3Xv3Cew.
- Smith D, Španěl P, Demarais N, Langford VS, McEwan MJ (2023). Recent developments and applications of selected ion flow tube mass spectrometry (SIFT-MS). Mass Spec. Rev. e21835. https://doi.org/10.1002/mas.21835.
- Španěl P, Smith D (2008). Quantification of trace levels of the potential cancer biomarkers formaldehyde, acetaldehyde and propanol in breath by SIFT-MS. J. Breath Res. 2, 046003. https://doi.org/10.1088/1752-7155/2/4/046003.
- US NTP. National Toxicology Program, U.S. Department of Health and Human Services (HHS) (2011). “12th Report on Carcinogens.” June 10, 2011.
This information has been sourced, reviewed and adapted from materials provided by Syft Technologies.
For more information on this source, please visit Syft Technologies.