Introduction
Solid fast-oxide conductors could be used as electrolyte materials for solid-electrolyte fuel cells, oxygen gas sensors and electrochemical pumps for oxygen separation. Yttrium-stabilized Zirconium is used in most mentioned applications of solid oxide electrolytes. However, one of its disadvantages is the operating temperature required for high conductivity of about >700°C.
Current solid oxide fuel cells (SOFCs) use yttrium stabilized zirconium (YSZ) as the electrolyte material which, however, must be operated at near 1000°C to achieve useful oxygen ion conductivity. This high temperature shortens the life span of the fuel cell and requires specialized materials for the other components especially the electrodes.
In recent years, low temperature oxide ion conducting systems have been the subject of a great deal of research. Abraham and Co-workers [1] have discovered high ion conductivity in Bi4V2O11. This compound exists in three polymorphic forms with transition temperatures:
|
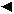 450°C 450°C
<———>
|
|
560°C
<———>
|
|
α
|
β
|
γ
|
Only the γ-phase has high oxide ion conductivity.
From a structural point of view, this compound can be formulated as (Bi2O2)2+(VO3.5 0.5)2-. The average V-O polyhedron appears as a squashed octahedron and the different oxygen deficient octahedral connected by sharing corners. On cooling, ordering of different V-O polyhedral and O-vacancies occurs leading to β-Bi4V2O11 and then α-Bi4V2O11. The α- and β-phase crystallize in the orthorhombic system and the γ-phase in tetragonal I4/mmm space group.
To stabilize the high temperature disordered γ-phase, Abraham and Co-workers [2] have imagined to prevent order by partial substitution of vanadium by aliovalent cations (di, tri, tetra). In some cases this leads to stabilisation of the γ-phase down to room temperature. The solid solutions formed are collectively known as BIMEVOX family.
Up to now, the BIMEVOX compounds are synthesized by conventional solid state method [3], which involves repeated grinding and firing of component oxides until a single phase material is achieved. It is difficult to control the phase homogeneity and purity, particle size and its distribution.
In our laboratory, we have synthesized complex oxides by sol-gel citrate method [4]. The sol-gel method offers several advantages with respect to conventional solid state such as low processing temperatures, high purity and homogeneity of the products and a wide range of obtainable materials. In this work, for the first time, we have synthesized some BIMEVOX compounds by the sol-gel citrate method and studied their electrical properties.
Experimental
The Bi(NO3)3.5H2O, V2O5, Co(CH3COO)2.4H2O, Cu(NO3)2.3H2O and NH3 used in this study were all analytical grade. Figure 1 illustrates a schematic diagram of Bi2MexV1-xO5.5-1.5x (Me = Co, Cu) synthesis.
|
|
|
Figure1. Schematic diagram of Bi2MexV1-xO5.5-1.5x (Me = Cu, Co) synthesis.
|
First Bi(NO3)3, Co(CH3COO)2 and V2O5 (in citric acid) solutions were mixed with molar ratio Bi3+:Co2+:V5+ = 1:x:(1-x). Citric acid (CA) aqueous solution was added into the above mentioned solution with molar ratio CA:∑Mn+ = 1:1. The obtained solution was vigorously stirred at room temperature. The pH of the solution was adjusted to 7-7.5 by adding ammonia solution. These conditions were drawn from our study of the influence of molar ratio CA:∑Mn+ and pH of solution on the citrate gel formation. By heating and vigorously stirring the solution at 70-80oC, a homogeneous gel was obtained. After drying in air at 80-90oC for a day, the gel was converted to a xerogel.
The thermal decomposition behaviour of the xerogel during heating was examined by means of differential thermal analysis (DTA) and thermogravimetric analysis (TGA) in dry air using thermal analyser TA2960-USA. The structure of powder products formed during heating was investigated using X-ray diffractometer Siemens D5005 (CuKα radiation) over the range of 2θ from 10o to 60o at angle step of 0.04o.
The electrical conductivity of the samples as determined by means of complex impedance spectroscopy in the frequency range from 5 Hz to 13 MHz using Impedance Analyser HP4192A.
Results and discussion
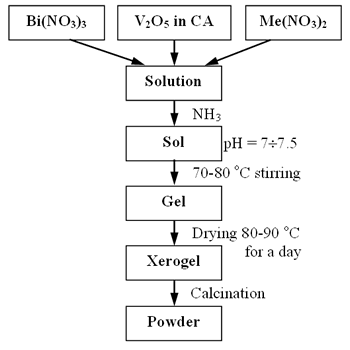
Figure 2 shows the DTA, TG curves of the gel (Bi3+:Co2+:V5+ = 1:0.1:0.9) after drying at 80-90oC for a day. DTA curve was performed from 25 to 800oC at a heating rate of 10o/min. Sharp and strong exothermic peaks observed at 207 and 254oC can be attributed to auto-redox of NH4NO3 and combustion of organic substances respectively. The TG curve shows a weigh loss of 65% in the temperature range 127-587oC.
|
|
|
Figure 2. DTA and TG curves of the gel (Bi3+:Co2+:V5+ = 1:0.1:0.9) after drying at 80-900C.
|
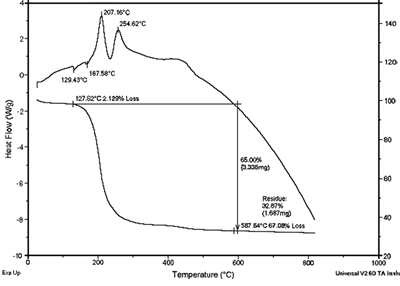
Figure 3 presents the X-ray patterns of xerogel heated at various temperatures. At low temperatures (under 600oC) the peaks (020) (d020 = 2.773 Å) and (200) (d200 = 2.730 Å) are separated. With increasing temperature, these peaks (α-phase) more approach (α ⇔ β transition Fig.3b, c, d) and finally coincide at 700oC (β ⇔ γ transition). Powder heated at 700oC for 2 h is single γ-phase (Figure 3e).
|
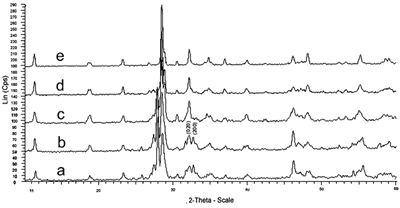
|
|
Figure 3. X-ray patterns of xerogel heated at various temperatures,a) 300oC; b) 400 oC; c) 500oC; d) 600oC; e) 700oC.
|
The crystalline phase formation (Bi2Co0.1V0.9O5.35) at low temperature (300oC-Figure 3a) indicates that by the citrate gel method described above, all elements are homogeneously mixed at the atomic level.
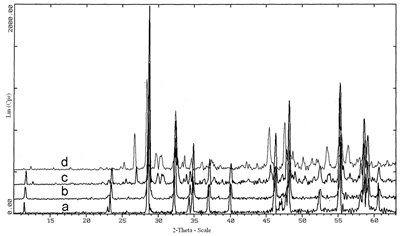
We have studied the influence of substitution of vanadium ions by copper ions on Bi2CuxV1-xO5.5-1.5x structures (Figure 4). The γ -phase only exists in the range of 0.07≤x≤0.2.
|
|
|
Figure 4. X-ray patterns of Bi2CuxV1-xO5..5 -1..5x , a) x = 0.07; b) x = 0.10; c) x = 0.20; d) x = 0.30.
|
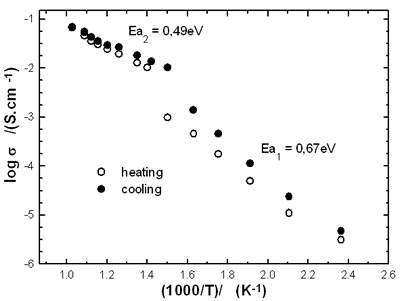
In order to evaluate the electrical property of the BIMEVOX electrolytes prepared by sol-gel citrate method, electrical conductivity measurements were performed on sintered pellets by means of complex impedance spectroscopy. The plots of logσ versus 103/T shown in Figure 5 represent data collected during the first heating and cooling cycles for Bi2Co0.1V0.9O5.35 (BICOVOX.10) in the temperature range 150oC to 700oC. Regarding the Arrhenius plots of this compound a classical evolution is observed: the conductivity behaviour shows two linear regions with different activation energies and the hysteresis loop is seen between heating and cooling cycles.
|
|
|
Figure 5. Temperature dependence of conductivity for Bi2Co0.1V0.9O5.35 prepared by sol-gel citrate method method.
|
For the BICOVOX.10 prepared by sol-gel citrate method, the activation energy of conductivity derived from the slope of the Arrhenius plot (for cooling cycle) has a value of Ea1 = 0.67 eV in the temperature range from 150oC to 400oC and Ea2 = 0.49 eV in the 400-700oC range. These values are slightly higher than ones obtained at the same x for BICOVOX prepared by the solid reaction method [5].
As a comparison, the conductivity and the activation energy values of the Bi2Co0.1V0.9O5.35 prepared by different methods are reported in Table 1.
Table 1. Conductivity and activation energy values of Bi2Co0.1V0.9O5.35
|
|
|
Conductivity at 300oC [S.cm-1]
|
6.10-4
|
3.1 x 10-3
|
|
Activation energy in the low temperature region [eV]
|
0.67
|
0.66
|
|
Activation energy in the high temperature region [eV]
|
0.49
|
0.39
|
The conductivity of BICOVOX.10 is lower in the case of material prepared by sol-gel method. This is due to the small density of the pellets.
By sol-gel method, the fine particles were often agglomerated on calcinations. The agglomerated strength has been determined by the extent to which water molecules and/or hydroxyl groups incorporated in the coordinated structure that are able to form strong oxygen bridges between adjacent particles. The hard agglomerates lead to incomplete densification.
In this report, we have used heterogeneous toluene azeotropic distillation to avoid the formation of hard agglomerate.
Table 2 lists the densities (%), conductivity and activation energy values of BICOVOX.10 before and after distillation.
Table 2. Densities (%), conductivity and activation energy values of Bi2Co0.1V0.9O5.35 before and after distillation.
|
|
|
Density [%]
|
72
|
88
|
|
Conductivity at 300oC [S.cm-1]
|
6.10-4
|
2.10-2
|
|
Activation energy in the low temperature region [eV]
|
0.67
|
0.42
|
|
Activation energy in the high temperature region [eV]
|
0.41
|
0.17
|
It is found that the density of BIMEVOX after distillation increases from 72% to 88% and its conductivity is higher than that synthesized by solid reaction by one order of magnitude.
Conclusions
We have synthesised Bi2MexV1-xO5.5-1.5x by the citrate gel method with high homogeneity and purity. The single γ-phase is obtained at a temperature lower than that prepared by conventional solid state method. By heterogeneous toluene distillation, the conductivity is higher than that synthesized by solid state method by one order of magnitude.
Acknowledgement
This research was completed with financial support from the National Science Council of Vietnam under code number 530201.
References
1. F. Abraham, M. F. Debreuille-Gresse, G. Mairesse and G. Nowogrochi, “Phase transitions and ionic conductivity in Bi4V2O11 an oxide with a layered structure“, Solid State Ionics, 28-30 (1988) 529-532.
2. F. Abraham, J.C. Boivin, G. Mairesse and G. Nowogrochi, “The bimevox series: A new family of high performances oxide ion conductors”, Solid State Ionics, 40-41 (1990) 934-937.
3. F. Krok, I. Abrahams, M. Malys, W. Bogusz, J. R. Dygas, J. A. G. Nelstrop and A. J. Bush, “Structural and electrical consequences of high dopant levels in the BIMGVOX system”, Solid State Ionics, 136-137 (2000) 119-125.
4. Nguyen Hanh, Huynh Dang Chinh and Nguyen Chau, “Synthesis of perovskite manganites La1-xSrxMnO3 by the sol-gel method“, Proceedings of the Third Vietnamese-German Seminar on Physics and Engineering, Institute of Engineering Physics-Hanoi University of Technology, (2000) 129-132.
5. Phan Quoc Pho and Vo Thach Son, “Electrical conductivity of BICOVOX electrolyte”, Proceedings of the Third Vietnamese-German Seminar on Physics and Engineering, Institute of Engineering Physics-Hanoi University of Technology, (2000) 71-74.
Contact Details
|