A research team from the Brown University has developed a triple-headed metallic nanoparticle catalyst for formic-acid fuel-cell reactions.
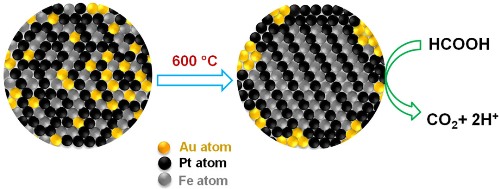 Gold atoms create orderly places for iron and platinum atoms, then retreat to the periphery of the fuel cell, where they scrub carbon monoxide from fuel reactions. The tighter organization and cleaner reactions extend the cell's performance life. (Credit: Sun Lab/Brown University)
Gold atoms create orderly places for iron and platinum atoms, then retreat to the periphery of the fuel cell, where they scrub carbon monoxide from fuel reactions. The tighter organization and cleaner reactions extend the cell's performance life. (Credit: Sun Lab/Brown University)
In experiments, a 4-nm iron-platinum-gold nanoparticle (FePtAu) catalyst generated a current of 2809.9 mA/mg Pt and retained a mass activity of 2600 mA/mg Pt or 93% of its initial mass activity even after 13 h. According to the team, the mass activity achieved by its tetragonal-crystal-structured trimetallic nanoparticle is better than that of all other nanoparticle catalysts. The research findings have been reported in the Journal of the American Chemical Society.
Gold plays a major role in the formic-acid fuel-cell reaction. It enables arrangement of the platinum and iron atoms as uniform layers inside the nanoparticle and then binds to the nanoparticle assembly’s exterior surface. Since the gold atoms form an additional space in the nanooparticle sphere initially, gold enables arrangement of iron and platinum atoms effectively. The gold atoms create more space for the platinum and iron atoms to self-assemble by diffusing from the space when heated.
Gold forms the necessary four-sided crystal structure dubbed as ‘face-centered-tetragonal’ at a lower temperature in the nanoparticle assembly. The four-sided shape helps in creating a more ordered structure by forcing the platinum and iron atoms to occupy specific positions in the structure. By applying an atomic order, the platinum and iron layers bind more strongly in the structure, resulting in a more stable and durable nanoparticle assembly, which is necessary for durable and high-performance catalysts. Gold also eliminates carbon monoxide from the formic-acid fuel-cell reaction by catalyzing its oxidation, thus enhancing the iron-platinum catalyst’s performance.
The research team noted that gold may be replaced with other metals in the nanoparticle catalyst to enhance durability and performance of the catalyst.