Apr 5 2019
NUS chemists have developed a cost effective method to synthesise geminal organodiboron compounds which are versatile chemical building blocks for the development of fine chemicals.
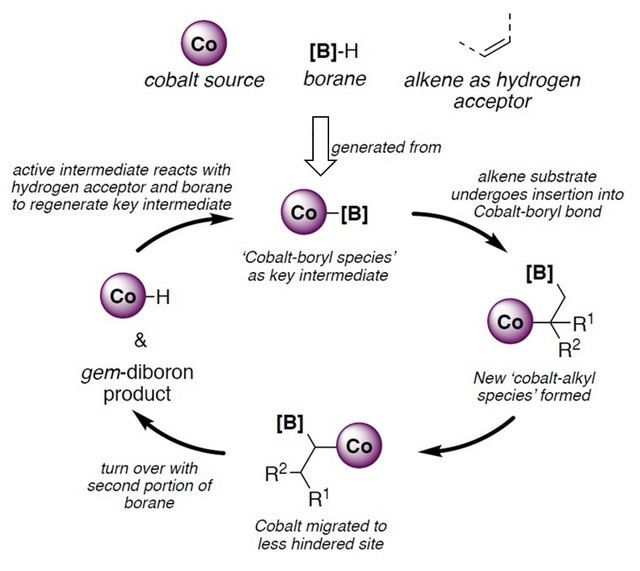 Figure 1: Schematic showing the proposed catalytic pathway for the synthesis of geminal organodiboron compounds.
Figure 1: Schematic showing the proposed catalytic pathway for the synthesis of geminal organodiboron compounds.
Organoboron compounds play an increasingly important role in organic chemistry due to their low toxicity and stability. These compounds contain active carbon-boron bonds which can readily undergo diverse chemical reactions to produce new chemical compounds with a wide variety of functional groups. A class of organoboron compounds, known as geminal organodiboron compounds, has emerged in recent years as synthetically versatile building blocks for a wide variety of applications. Geminal organodiboron compounds refer to organic molecules with two boron atoms that are bonded to the same carbon atom. However, geminal organodiboron compounds are difficult to obtain due to a lack of established synthetic methods to produce them and the scarcity of the starting materials.
Prof GE Shaozhong and his research group from the Department of Chemistry, NUS have developed a method to synthesise highly enantioselective geminal organodiboron compounds from alkenes and boranes which are both readily available chemicals. This reaction is achieved by using a cobalt catalyst, an inexpensive base metal (figure 1). Chiral molecules exist in pairs (known as enantiomers) and are mirror images of each other, similar to the relationship between our left and right hands. This method is able to produce geminal organodiboron compounds of one of these molecular pairs with high yield. The two enantiomers in general have different bioactivity, and the selective production of one enantiomer is important for the development of medicinal drugs.
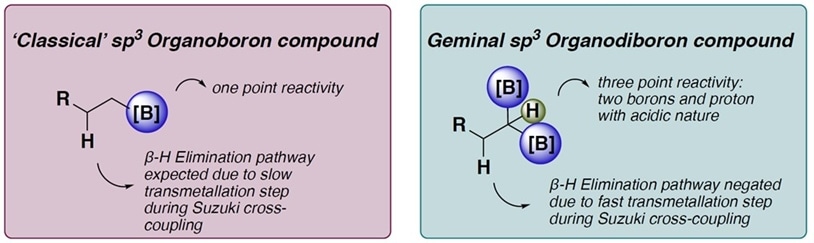
Figure 2: Comparison of the reactivity between “classical” organoboron compound (on the left) and geminal organodiboron compound (on the right).
Compared to classical organoboron reagents, geminal organodiboron reagents have better reactivity for chemical synthesis due to the arrangement of the molecule (figure 2). The use of such reagents could potentially improve the production efficiency of fine chemical products.
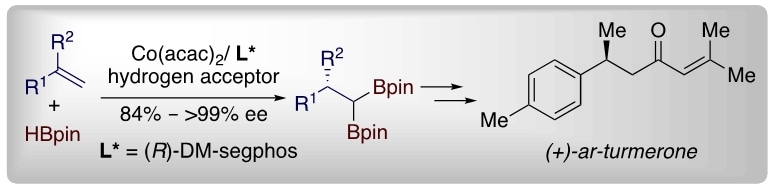
Figure 3: Schematic showing the synthesis of (+)-ar-turmerone, a compound with known bioactive properties found in the rhizome of the turmeric plant.
Prof Ge said, “The geminal organodiboron compounds developed from this method can be readily converted into a variety of chiral molecules and natural products by standard functional group manipulations. As an example, our team has used this method to synthesise a bioactive compound commonly found in turmeric plants (figure 3).”
The group plans to extend the method to develop other synthetic methods involving novel multi-boron containing compounds.
References
Teo WJ; Ge SZ*, "Cobalt-catalyzed enantioselective synthesis of chiral gem-Bis(boryl)alkanes" ANGEWANDTE CHEMIE-INTERNATIONAL EDITION Volume: 57 Issue: 39 Pages: 12935-12939 DOI: 10.1002/anie.201805705 Published: 2018.
Teo WJ; Ge SZ*, "Cobalt-catalyzed diborylation of 1,1-disubstituted vinylarenes: A practical route to branched gem-Bis(boryl)alkanes" ANGEWANDTE CHEMIE-INTERNATIONAL EDITION Volume: 57 Issue: 6 Pages: 1654-1658 DOI: 10.1002/anie.201710389 Published: 2018.