Water has a fundamental role to play in the existence of humans and is a huge component of our universe, yet there are still many things that are not clear about it.
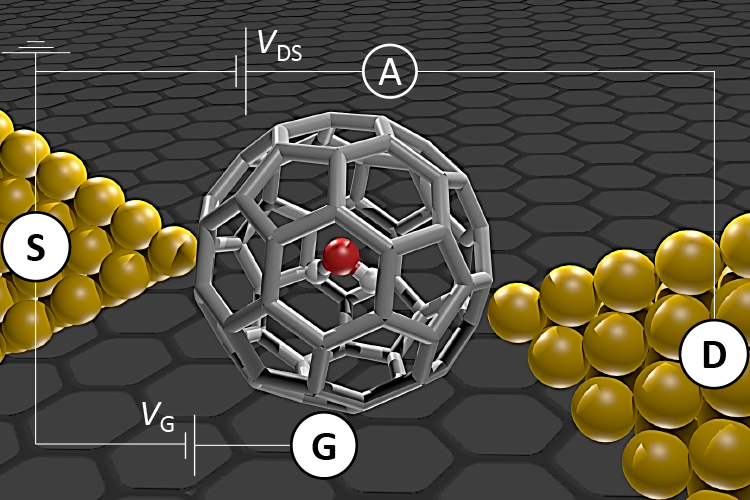
Image Credit: Institute of Industrial Science, University of Tokyo.
To find answers and fill the knowledge gaps, a collaborative team of the Institute of Industrial Science, the University of Tokyo, Kyoto University and Tohoku University explored electron flow through a single water molecule in a C60 cage. Their findings have been published in the journal Nano Letters.
Basic systems are generally the ideal starting point for establishing complex information. A single water molecule is an example of such a system. Composed of only three atoms, it offers an outstanding model for determining quantum mechanical information.
Adding a water molecule into a C60 cage — a soccer-ball-shaped molecule composed completely of carbon atoms — forms H2O@C60 and is an exceptional way of isolating water for analysis. The scientists realized this using "molecular surgery", which requires opening the cage, adding water and closing the cage again.
H2O@C60 was then employed as a single-molecule transistor (SMT) by placing one H2O@C60 molecule in the tiny gap — less than 1 nm — between two gold electrodes. Since the electric current then travels via the isolated molecule only, the electron transport can be examined with high precision.
A conductance map, also called a "Coulomb stability diagram", was produced for the H2O@C60 SMT. It displayed numerous tunneling-induced excited states for the water molecule. In comparison, the Coulomb stability diagram of an empty C60 cage SMT displayed just two excited states.
Because it contains two hydrogen atoms, water has two different nuclear spin states: ortho- and para-water. In ortho-water, the hydrogen nuclear spins are in the same direction, while in para-water they are opposite to one another. Understanding the transition between these two types of water is an important area of research.
Shaoqing Du, Study Lead Author, Institute of Industrial Science, University of Tokyo
The team measured the tunneling spectra for the H2O@C60 system, and by comparing the outcomes with theoretical calculations, was able to credit the measured conductance peaks to vibrational and rotational excitations of the water molecule. They also examined H2O@C60 using terahertz spectroscopy and the results matched with the tunneling spectroscopy data.
Both methods demonstrated quantum rotational excitations of ortho- and para-water concurrently. This shows that the single water molecule transitioned between the two nuclear isomers (ortho- and para-water) within the period of the experiment, which was around 1 minute.
Our findings make an important contribution to the understanding of ortho-para fluctuation in water molecules. Because water plays such an important role in chemistry and biology, and even in understanding our universe, we expect our findings to have a wide-ranging impact.
Kazuhiko Hirakawa, Study Corresponding Author, Institute of Industrial Science, University of Tokyo
Journal Reference:
Du, S., et al. (2021) Inelastic Electron Transport and Ortho–Para Fluctuation of Water Molecule in H2O@C60 Single Molecule Transistors. Nano Letters. doi.org/10.1021/acs.nanolett.1c03604.