Growing energy demand and the threat of climate change-induced ecological damage have increased the interest of researchers and governments in alternative, green energy storage technologies to address the damage caused by the use of greenhouse gas-emitting fossil fuels. Technological solutions are central to achieving net carbon neutrality by 2050, which has been set as a target by international bodies and governments.
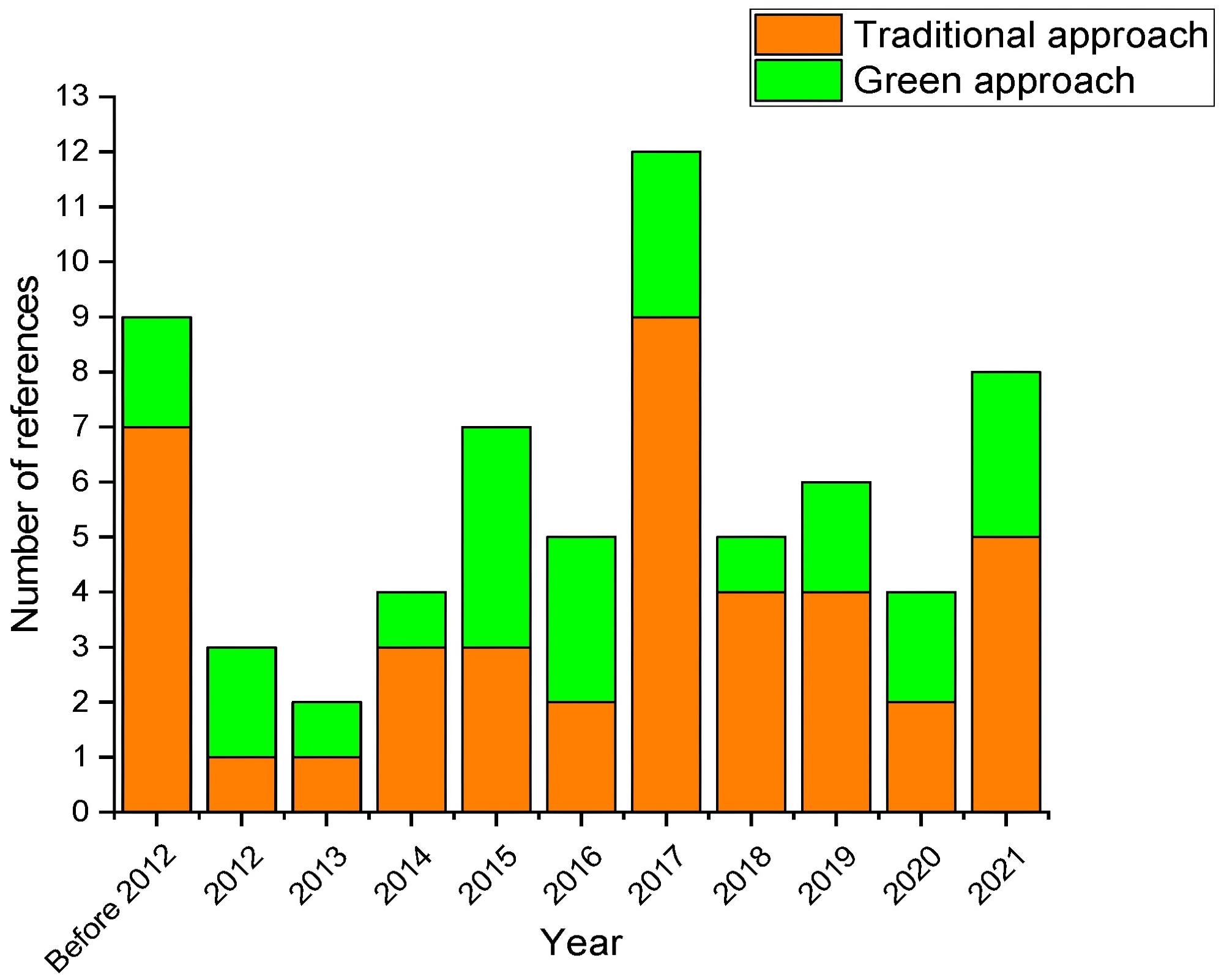
Chronological distribution of the scientific papers selected for this review. Image Credit: Mas, A.B et al., Sustainability
Amongst these technologies, fuel cells have been explored in recent years due to their superior electrical efficiency, low pollution emissions, and reduced noise. Compared to other types of fuel cells, solid oxide fuel cells possess longer durability and 60-80% higher conversion efficiency.
Solid oxide fuel cells and solid oxide electrolyte cells are jointly referred to as solid oxide cells. These two related technologies have different proposed applications: solid oxide fuel cells have been proposed as an alternative to conventional combustion technologies to produce electricity, whilst solid oxide electrolyzer cells have potential for use in the conversion of steam to hydrogen.
Solid oxide cells are constructed of porous anodes and cathodes separated by a dense layer of electrolytes. Common materials used in the construction of solid oxide cells include metals such as nickel, copper, strontium, and cobalt, and rare earth elements such as yttrium, lanthanum, and cerium. Due to their critical economic importance and scarcity, cobalt and rare earth elements were classified as critical raw materials by the European Union in 2020.
Solid oxygen fuel cells have been widely investigated for distributed stationary power generation, and the global market for these technologies is predicted to reach $5.31 billion by 2028. However, there are still challenges associated with this technology, notably the exploitation of finite natural resources. This has facilitated research into efficient and cost-effective recycling strategies for solid oxide cell waste streams.
Waste Valorization Strategies
Many waste valorization strategies have been proposed and investigated by researchers to reduce the economic and environmental impact of exploiting critical raw materials such as cobalt and rare earth elements, including lanthanum.
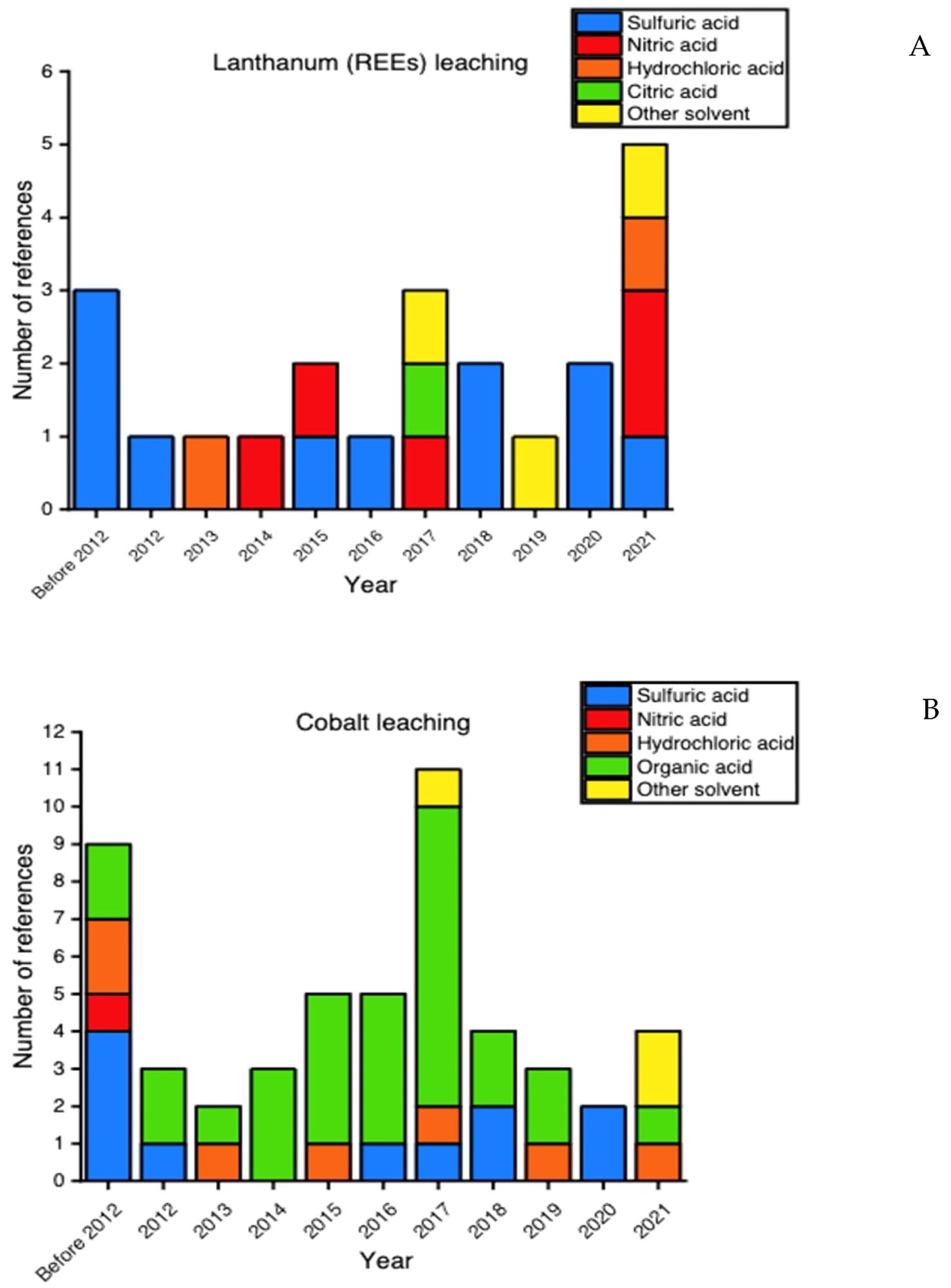
Type of solvents considered for (A) lanthanum and (B) cobalt leaching in the scientific papers selected for this review. Image Credit: Mas, A.B et al., Sustainability
In the past decade, strategies have been widely investigated for recycling valuable end-of-life waste streams such as e-waste and spent catalysts and batteries. Different-scaled approaches including pyrometallurgy and hydrometallurgy and liquid-liquid solvent extraction, precipitation, filtration, and bioleaching have been proposed.
Each process is based on different principles and has distinct advantages. Amongst these approaches, sustainable hydrometallurgical processes which use mild solvents and limit emissions and energy demands have shown particular promise and high recovery rates. However, there has been a lack of specific definition on what is intended for “green” processes for the recovery of critical raw materials.
The Study
Due to their economic value and wide application in a variety of industries, cobalt and lanthanum are critical resources. The study has investigated the use of leaching to recover these elements from waste streams to improve the sustainability and circularity of solid oxide cell production.
The authors have stated that despite the recognized importance of recycling end-of-life solid oxide cell materials, currently there is a lack of research on this topic. Current studies have concentrated on the recovery of waste materials from different types of fuel cells. Additionally, whilst there have been studies that have proposed efficient processes for the recovery of cobalt and lanthanum, these studies concentrate on different waste streams than fuel cells.
Leaching is the first phase of the hydrometallurgical waste recovery process. The authors have provided a review of studies in the current literature on the recovery of cobalt and lanthanum using leaching from a variety of sources such as e-waste, spent catalysts, and waste batteries. Both these elements are present in the perovskite structure of solid electrolytes, which facilitates the need for an efficient recycling process for these elements from solid oxide cells.
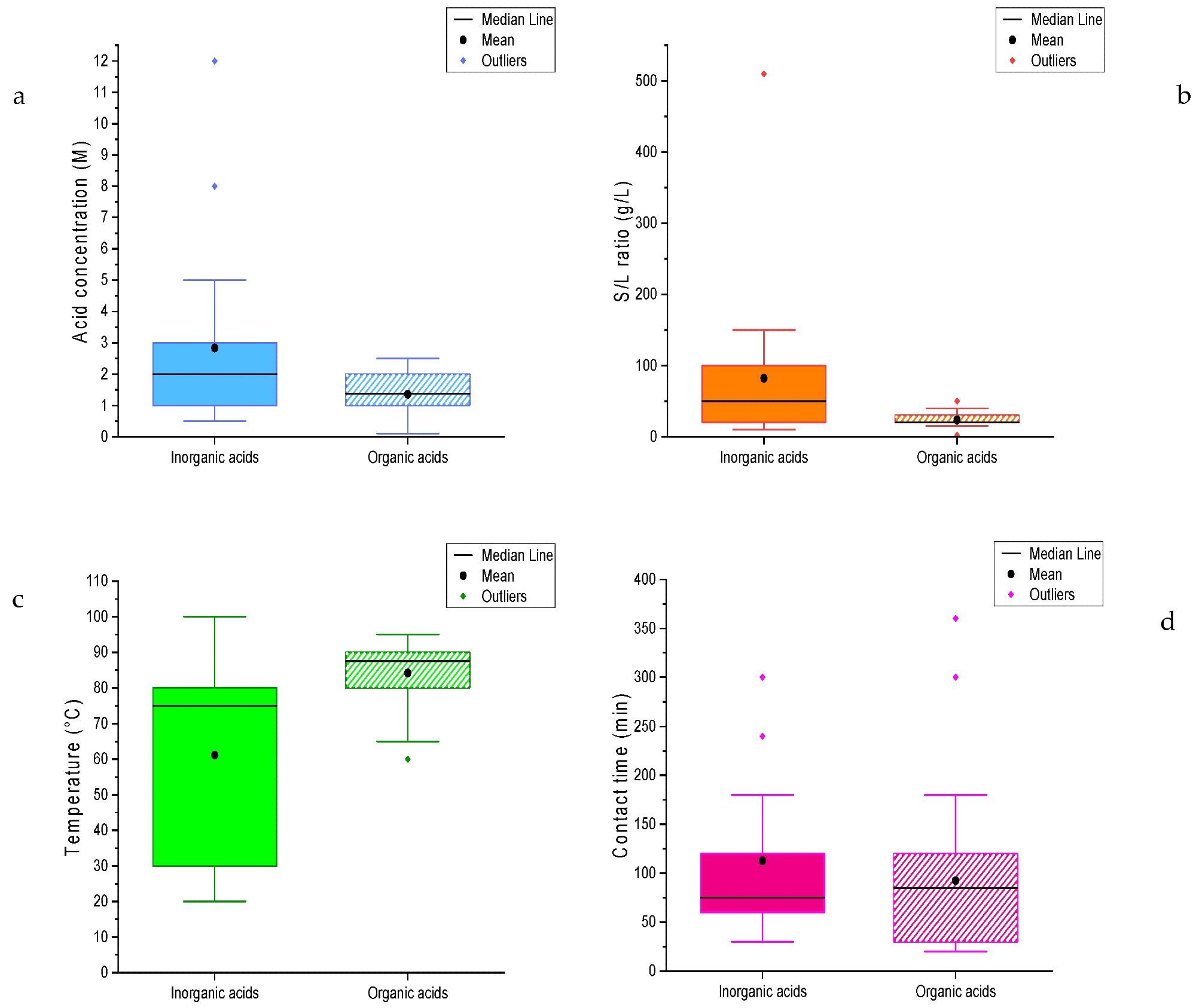
Box plots of the values of the experimental parameters for the recovery of cobalt: (a) Acid concentration; (b) S/L ratio; (c) Temperature; (d) Contact time. Image Credit: Mas, A.B et al., Sustainability
Additionally, the authors have evaluated current processes with the aim of finding suitable “green” processes. They evaluated the degree to which processes adhere to green chemistry principles, namely less hazardous chemical syntheses, designing safer chemicals, using safer solvents and auxiliaries, design for degradation, and the use of inherently safer chemistry to prevent accidents. Thus, the safety of these processes and their potential to reduce environmental damage were analyzed. Processes were declared to be green if they adhered to three of the five principles.
Based on their literature study, the authors have proposed that a process for successful, green, and efficient cobalt and lanthanum recovery from waste solid oxide cell materials could be based on an acid concentration of 1.5-2 M, a 20-100 g/L solid-liquid ratio, temperature of 70-80oC, and a contact time of eighty to one hundred and eighty minutes. This is irrespective of the solvent used in the process. Given the urgency of recovering these elements from waste solid oxide cell materials, the authors have stated that their study can provide some useful hints for future research.
Further Reading
Mas, A.B et al. (2022) Analysis of Lanthanum and Cobalt Leaching Aimed at Effective Recycling Strategies of Solid Oxide Cells [online] Sustainability 14(6) 3335 | mdpi.com. Available at: https://www.mdpi.com/2071-1050/14/6/3335
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.