Silk cultivation has been carried out for centuries with the help of domesticated silkworms. However, it has been hard to commercially bulk produce spider silk as a result of their cannibalistic tendencies.
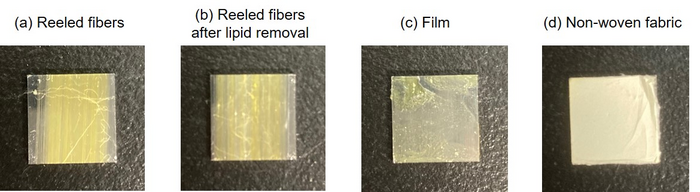 Appearance of the native spider silk samples on stainless steel plate: (a) reeled fibers, (b) reeled fibers after removal of lipid layers, (c) films, and (d) non-woven fabrics. Image Credit: Reprinted with Permission from Langmuir 2022, Copyright (2022) American Chemical Society.
Appearance of the native spider silk samples on stainless steel plate: (a) reeled fibers, (b) reeled fibers after removal of lipid layers, (c) films, and (d) non-woven fabrics. Image Credit: Reprinted with Permission from Langmuir 2022, Copyright (2022) American Chemical Society.
Spider silk fibers, on the other hand, are gaining popularity due to their fineness, mechanical characteristics, and lustrous appearance. Spider silk produced via recombinant protein expression systems and chemical synthesis has been shown to possess excellent properties for medical use.
This helps in preventing the development of blood clots and has outstanding knot strength to bear repetitive loading and unloading. In this study, an analysis was done on the cell adhesion behavior of native spider silk.
The cell culture substrates’ development seems to be essential for the advancement of regenerative medicine. As far as conventional research is concerned, cell culture substrates made from petroleum-derived polymers have been developed. However, the development of protein-derived cell culture substrates has not made much progress.
Since ancient times, amongst the protein-based materials, silkworm silk has been utilized. Over the past few years, attention has been concentrated on spider-derived silk, which possesses improved mechanical properties compared to silkworm silk.
But not a lot is known regarding cell behavior on spider silk. Hence, for this study, scientists headed by Dr. Kenjiro Yazawa of Shinshu University aimed to examine the cell adhesion behavior on spider silk.
In earlier studies, experiments were conducted along with recombinant spider silk-like proteins rather than natural spider silk. Hence, the size of the protein was nearly 1/10th of that of natural spider silk.
The research group involves Dr. Jun Negishi, an expert in biomaterials who thought that it was crucial to gather spider silk directly from live spiders and note the cell adhesion of natural spider silk.
Three kinds of spider silk; film, reeled fibers, and nanofiber (non-woven fabric), were made by the scientists. It was difficult to wind live spider thread so that it would be oriented in a similar direction. But they achieved this and they discovered that there was a variance in the shape of cell adhesion among the three shapes of spider silk (TOC Graphic).
This study verified the adhesion behavior of fibroblasts on spider silk, but it is still essential to examine if there is a variation in cell activity based on the surface topography. For instance, if the cell activity is high on a thread or non-woven fabric or that it is low on film, it will be a new breakthrough. At present, this concept of native spider silk is under investigation.
The study was funded by JSPS Grant-in-Aid for Scientific Research (C) grant no. 21K12305.
Journal Reference:
Yazawa, K., et al. (2022) Cell Adhesion Behaviors on Spider Silk Fibers, Films, and Nanofibers. Langmuir. doi.org/10.1021/acs.langmuir.2c00818.