Since the beginning of the COVID-19 pandemic in early 2020, the scientific community has focused its efforts on developing strategies to prevent the spread of SARS-CoV-2 in the general population.
Aside from the development of vaccines against SARS-CoV-2, there have been two main strategies for mitigating the spread of the virus which causes COVID-19: social distancing and face masks. In response to the pandemic, governments mandated the employment of both strategies in public spaces. Both strategies have been extremely effective in stopping the transmission of respiratory viruses such as SARS-CoV-2.
Early in the pandemic, there was a critical shortage of disposable, medical-grade surgical masks for use by the general public to stop the spread of COVID-19. An initial solution was the use of washable and reusable cloth masks, either commercially produced or homemade. Studies and mathematical modeling confirmed the efficaciousness of face masks.
The ability of a face mask to stop respiratory viruses depends on its filtration performance for particles in the range of 1-3 μm in diameter. This performance varies significantly with the material used in face masks. Most common textiles can filter 100% of particles above 3 μm in diameter, blocking any that come into contact with the mask.
However, concerns have been raised about self-contamination due to a lack of knowledge among the general population about safe mask use. The risk of self-contamination due to unsafe handling practices when individuals put masks on or take them off severely offsets the benefits of using face masks.
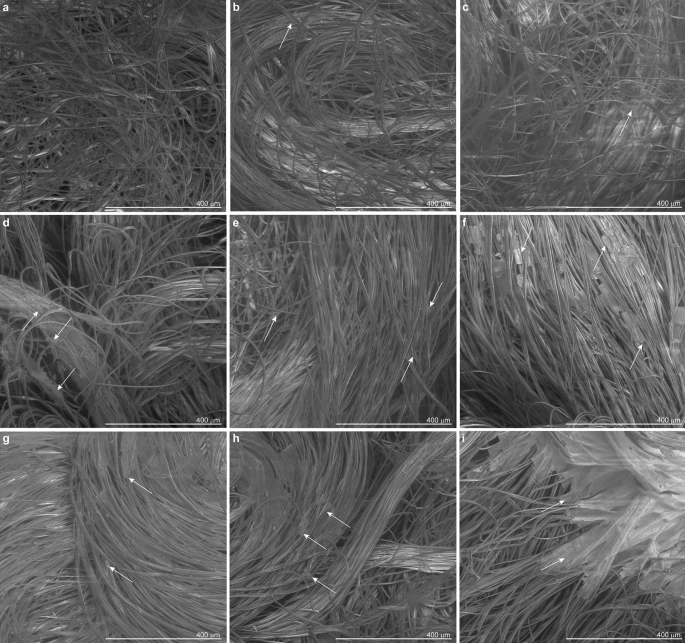
Scanning electron microscopic images of test material, salt-coated by spray and dip methods. The salt formulation was used non-diluted or diluted. Spray treatments allowed the deposition of increasing amounts of salt by varying the valve aperture of the spray device according to arbitrary stroke units. (a) Non-coated, (b) spray, undiluted salt formulation, stroke unit 1 (Spr S1), (c) spray, fivefold diluted salt formulation, stroke unit 3 (Spr S3 Dil5 ×), (d) spray, undiluted salt formulation, stroke unit 3 (Spr S3), (e) spray, undiluted salt formulation, stroke unit 5 (Spr S5), (f) spray, undiluted salt formulation, stroke unit 10 (Spr S10). For dip treatments test materials were immersed into (g) tenfold diluted (Dip Dil10 ×), (h) fivefold diluted (Dip Dil5 ×), and (i) undiluted (Dip No Dil) salt formulations. Arrows on images point at examples of salt crystals and aggregates found on the test materials. Image Credit: Schorderet Weber, S., et al., Scientific Reports
Anti-Viral Coatings: A Strategy to Improve Face Masks
Improving the anti-viral properties of face masks to avoid indirect viral transmission has become a key priority in research since the beginning of the pandemic, with cost-effective and non-toxic anti-viral coatings emerging as a key solution.
Several coatings have been evaluated for their ability to improve the anti-viral properties of common face mask materials. Metals, metal oxides, carbon-derived materials, photoreactive materials, antimicrobial polymers, and biomolecules have been extensively researched by scientists.
One particularly interesting research direction is the use of sodium chloride to coat face masks. Sodium chloride, or common salt, is non-toxic, inexpensive, easily obtainable, and sustainable. Moreover, it can be employed in non-professional environments to produce commercial anti-viral masks as well as in home settings.
Studies have produced noteworthy results. Quan et al. produced anti-viral coatings for surgical face masks using melt-blown polypropylene with salt. The team reported a stable and efficient coating that inactivated H1N1 Influenza particles within five minutes due to physical disruption of capsids. Upon wetting with virus-laden aerosols, local dissolution and recrystallization of the salt coating occurred, which led to this physical disruption.
Inactivation of capsids occurred even under harsh conditions. Later studies by the same team further demonstrated the anti-pathogenic functionalization of inert membranes using sodium chloride coatings.
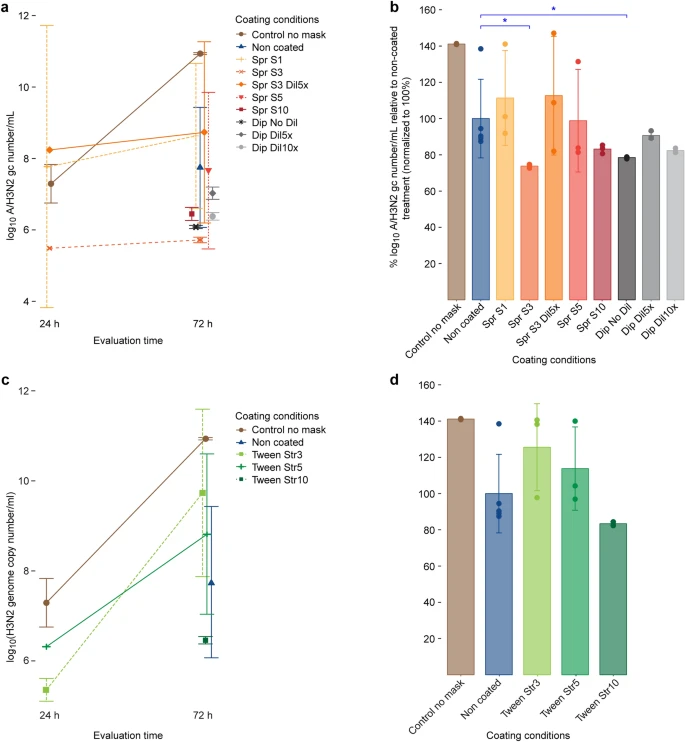
TAQMAN reverse transcription-polymerase chain reaction for the determination of A/H3N2 virus genome copy (gc) numbers in the apical medium of MUCILAIR epithelia infected after direct contact of the virus with a non-coated test material or a test material coated with various concentrations of salt or Tween. (a) Salt coatings, log10 A/H3N2 gc number/mL quantified at 24 and 72 h post infection. (b) Salt coatings, viral replication at 72 h post infection. (c) Tween coatings, log10 A/H3N2 gc number/mL quantified at 24 and 72 h post infection. (d) Tween coatings, viral replication at 72 h post infection. Data are expressed as a percentage relative to the mean of log10 A/H3N2 virus gc number/mL quantified in the infected non-coated treatment group, which was normalized to 100%. Means ± standard errors are shown; n = 3 in all treatments, except for non-coated (n = 5). *P < 0.05. Image Credit: Schorderet Weber, S., et al., Scientific Reports
The Paper
The new paper aims to further evaluate and confirm the efficaciousness of salt coatings for this critical application. The authors have noted that reusable face masks need to be regularly washed, so the anti-viral coating will need to be reapplied after each wash. Therefore, the research evaluates whether methods such as spraying and dipping and common fabrics will influence the anti-viral properties of coatings.
The test fabric selected for the study was a universal household cleaning cloth. This was coated with incremental salt concentrations using automated controlled spraying and dipping methods. Coatings conditions prior to viral exposure were assessed, with the research considering the deposited salt amount, distribution, and crystal size.
Eight coating conditions were tested. In vitro bioassays of influenza A H3N2 virus were used to evaluate and compare coating anti-viral properties in a 3D culture model of lung airway epithelial tissue.
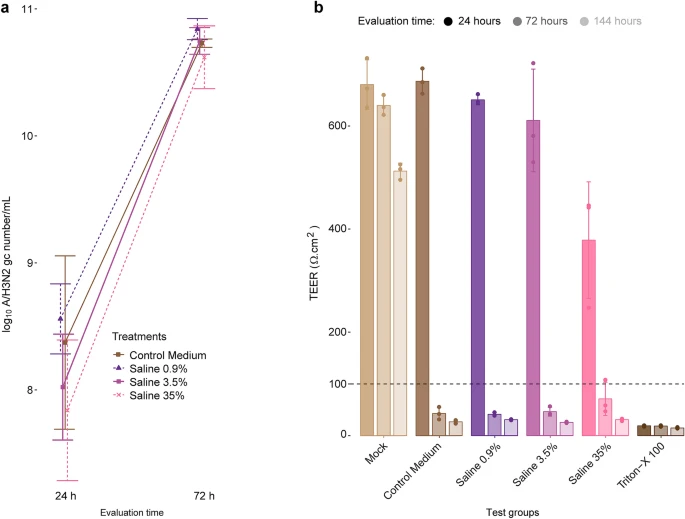
Effect of virus pre-incubation in hypertonic salt solutions on viral replication in cells. (a) TAQMAN reverse transcription-polymerase chain reaction for the determination of A/H3N2 virus genome copies (gc) in the apical medium of MUCILAIR epithelia infected after incubation of the virus in salt solutions of various concentrations. Data are expressed as log10 A/H3N2 gc number/mL, quantified at 24 and 72 h post infection. Means ± standard errors are shown; n = 3. (b) Transepithelial electrical resistance (TEER) measurements in MUCILAIR epithelia 24, 72, and 144 h post infection. Means ± standard errors are shown; n = 3. The dotted line represents the 100 Ω·cm2 limit of tissue integrity. Mock, non-infected non-treated control culture; Triton X-100, detergent used as a positive control. Image Credit: Schorderet Weber, S., et al., Scientific Reports
Study Findings
SEM micrographs of the test materials revealed a distributed crystal scatter on fabric fibers using both spray and dip-coating methods following deposition and evaporation.
Compared to non-coated fabrics, salt deposits of 4.3 mg/cm2 or above reduced viral replication on the test fabrics markedly. However, anti-viral properties are largely dependent on crystal distribution and size, even for large salt quantities, and these, in turn, depend on the coating technique.
Overall, the study has demonstrated the suitability of salt coatings for improving the anti-viral properties of face masks and confirmed the results of previous research in this area. The use of salt coatings can significantly mitigate the risk of self-contamination, but particular attention should be paid to coating protocols for consumer applications.
Further Reading
Schorderet Weber, S., et al. (2022) In vitro testing of salt coating of fabrics as a potential anti-viral agent in reusable face masks. Scientific Reports 12, 17041 [online] nature.com. Available at: https://doi.org/10.1038/s41598-022-21442-7
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.