Image Credit: University of Delaware
However, while developing processes to better understand the role of oxygen in the performance of carbon-based catalysts, Dion Vlachos of the University of Delaware and associates from the Catalysis Center for Energy Innovation (CCEI), along with collaborators from Brookhaven National Laboratory, made some unexpected discoveries that were reported in a study that was published in Nature Communications.
Vlachos claimed that some of their knowledge of chemistry had been turned on its head by what they had discovered.
Not all oxygens are the same.
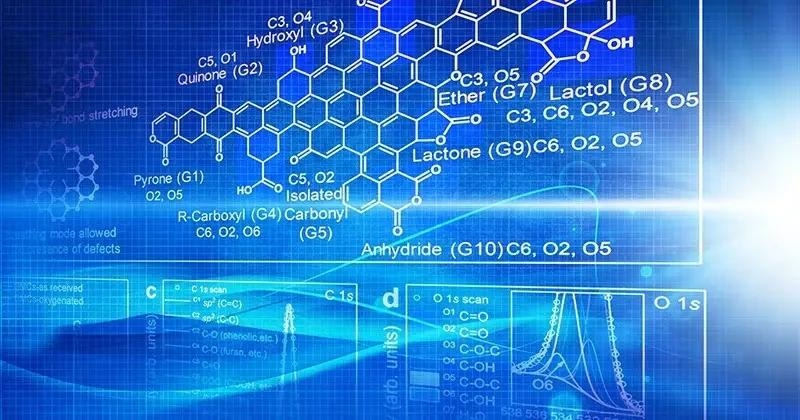
Carbons, despite their utility, are poorly understood. They are also inconsistent. Oxygen can appear as an alcohol, aldehyde, ketone, or acid in carbon-based materials on occasion. One unanswered question is what oxygen does in these carbon materials.
So, Vlachos and his colleagues took carbon molecules and gradually added more and more oxygen, then characterized the resulting material using spectroscopic techniques to determine how much and what type of oxygen was present.
The researchers did this for a library of 10 to 15 materials before performing reactions with the various oxygenated carbons. Using machine learning tools, they were able to correlate the reactivity of the carbon material with the amount and type of oxygens present.
The research team discovered a link between the amount and type of oxygen present and performance and which oxygens are more active. The researchers also found something unexpected: long-range effects from aromatic rings far from a catalyst site often can cause carbon alcohol groups to become more acidic than commonly associated acidic carbon functional groups present in organic chemistry small acids.
The researchers were initially surprised, but after some calculations, they confirmed that the effect was caused by alcohol-based oxygenated carbons in aromatic rings.
Carbon has aromatic rings. And the more carbon rings that are added to a material, the greater the chance of creating a regional phenomenon where long-range effects from far away can have a controlling effect on the activity of the catalyst sites.
Dion Vlachos, University of Delaware
Vlachos is also the Unidel Dan Rich Chair in Energy and the Director of CCEI, an Energy Frontier Research Center supported by the US Department of Energy.
This is not the case with conventional catalysis chemistry, where the effect is very local. Bond A, for example, has no effect on Bond B.
“The whole chemistry thinking is upside down. This was not expected,” he adds.
Vlachos explained that if investigators would like to produce a more acidic carbon catalyst, they would have to use more alcohol functional groups, specifically hydroxyls.
The investigators used sophisticated methods to validate the mathematical modeling results and characterize what happened to oxygen in materials under near-real-world conditions while the chemistry was actually occurring.
The University of Delaware team accomplished an impressive feat by using advanced tools and methods to unravel a complicated catalytic system. We are delighted to have played a part in this significant achievement by conducting measurements using a special kind of spectroscopy at the Center for Functional Nanomaterials.
Anibal Boscoboinik, Materials Scientist, Center for Functional Nanomaterials
Center for Functional Nanomaterials is a US Department of Energy (DOE) Office of Science User Facility at DOE’s Brookhaven National Laboratory.
The research group can test various techniques for making materials to see which approach has the best effect using this new approach to determine what each part of the chemistry is doing. For instance, are all oxygen molecules equally good at hastening catalytic reactions, or are some more effective than others?
Vlachos is also intrigued by the possibility of using the oxygen source to disperse metals for reactions. Standard methods for introducing oxygen into a reaction to make materials are corrosive, so discovering greener alternatives could bring more sustainable processes closer to reality.
Journal Reference:
Zhou, J., et al. (2023). Tuning the reactivity of carbon surfaces with oxygen-containing functional groups. Nature Communications. doi.org/10.1038/s41467-023-37962-3.