A newly developed ceramic filler may help alleviate the limitations of composite solid-state electrolytes. The filler not only mitigates interface barriers between the composite components but also provides an additional lithium-ion transport pathway, increasing the number of ions and the speed with which they move through the electrolyte. Image Credit: Energy Materials and Devices, Tsinghua University Press
However, it is important to note that lithium-ion batteries carry the risk of explosions. They consist of negatively and positively charged electrodes and an electrolyte that facilitates the movement of ions between them.
The performance of lithium-ion batteries is constrained by the characteristics of their individual components. For instance, liquid electrolytes can become volatile when exposed to high temperatures, and the overall efficiency of these batteries can be affected by non-uniformity and instabilities in other components.
Scientists are analyzing ways to create safer, more efficient batteries with solid electrolytes that can make a significant change over the liquid version transporting ions in most commercially available batteries.
According to the research by scientists based at the Shenzhen All-Solid-State Lithium Battery Electrolyte Engineering Research Center in Tsinghua Shenzhen International Graduate School’s Institute of Materials Research, the solid-state material has advantages and disadvantages.
To overcome the problem, the scientists combined two prime solid-state candidates, such as ceramic and polymer, into a new compound electrolyte.
They published their outcomes on September 21st, 2023, in Energy Materials and Devices.
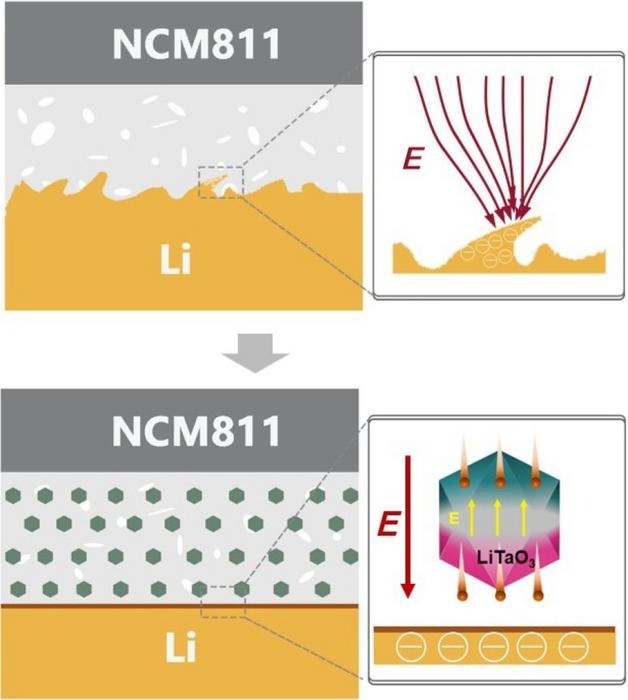
Composite solid-state electrolytes have received significant attention due to their combined advantages as inorganic and polymer electrolytes. However, conventional inorganic ceramic fillers offer limited ion conductivity enhancement for composite solid-state electrolytes due to the space-charge layer between the polymer matrix and ceramic phase.
Yu Yuan, Co-First Author, Tsinghua Shenzhen International Graduate School
Inorganic ceramic electrolytes are tough to manufacture and develop resistance when faced with another solid, though they offer high conductivity. Polymer electrolytes' conductivity at room temperature is too low; however, they are easy to produce, more flexible, and work better with electrodes, which are used in commercial applications.
Yuan says that combining the two should produce a highly conductive, flexible electrolyte that is simple to manufacture. The composite solid-state electrolytes have a separation called a space-charge layer between their constituent parts, limiting their conductivity.
To overcome this, the scientists used lithium tantalate, which has a crystalline structure that offers unique optical and electrical properties, as a functional filler to mitigate the space-charge layer. The ceramic ion conductor material is ferroelectric, which can reverse electric charge when current is applied.
Not only does the filler alleviate the space-charge layer, but it also provides an extra lithium-ion transport pathway.
Likun Chen, Co-First Author, Tsinghua Shenzhen International Graduate School
The scientists experimentally illustrated that the lithium tantalate filler mitigates the block for lithium-ion transport across the polymer-ceramic interface, resulting in lithium ions moving in both increased numbers and speed through the electrolyte.
Scientists conclude that an electrolyte with high conductivity and a long life cycle indicates the transport of ions across the battery in charging and discharging cycles at low temperatures.
This work proposes a novel strategy for designing integrated ceramic fillers with ferroelectric and ion-conductive properties to achieve high-throughput lithium-ion transport of composite-solid electrolytes for advanced solid-state lithium metal batteries. Our approach sheds light on the design of functional ceramic fillers for composite solid-state electrolytes to effectively enhance ion conductivity and battery performance.
Yu Yuan, Co-First Author, Tsinghua Shenzhen International Graduate School
Co-authors include Yuhang Li, Xufei An, Jianshuai Lv, Shaoke Guo, Xing Cheng, Yang Zhao, Ming Liu, Yan-Bing He, and Feiyu Kang. Li, An, Lv, Guo, Cheng, Zhao, and Kang are also affiliated with Tsinghua Shenzhen International Graduate School.
The National Natural Science Foundation of China, Key-Area Research and Development Program of Guangdong Province, Shenzhen Outstanding Talents Training Fund, All-Solid-State Lithium Battery Electrolyte Engineering Research Center, and Shenzheng Technical Plan Project funded the study.
Journal Reference
Yuan, Y., et al. (2023). Functional LiTaO3 filler with tandem conductivity and ferroelectricity for PVDF-based composite solid-state electrolyte. Energy Materials and Devices. doi.org/10.26599/EMD.2023.9370004