Researchers at Massachusetts Institute of Technology (MIT) have quantified lithium intercalation rates across various battery materials and utilized this information to create a novel model explaining the control of the reaction. The study was published in Science.
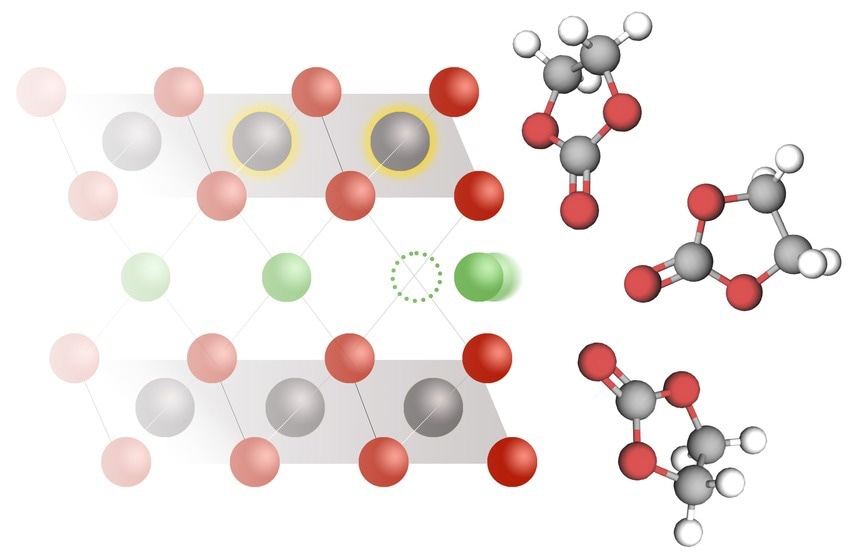 Lithium intercalation is the process by which lithium ions insert themselves into the solid electrode of a lithium-ion battery. MIT researchers have shown that as lithium ions (green) move from an electrolyte solution (right) to a cobalt oxide electrode (left), electrons also move into the electrode and reduce the cobalt (gray atoms with gold halo). Image Credit: Christine Daniloff, MIT
Lithium intercalation is the process by which lithium ions insert themselves into the solid electrode of a lithium-ion battery. MIT researchers have shown that as lithium ions (green) move from an electrolyte solution (right) to a cobalt oxide electrode (left), electrons also move into the electrode and reduce the cobalt (gray atoms with gold halo). Image Credit: Christine Daniloff, MIT
At the core of every lithium-ion battery lies a fundamental reaction: Lithium ions, which are dissolved in an electrolyte solution, "intercalate" or embed themselves into a solid electrode during the battery's discharge phase. When these ions de-intercalate and move back into the electrolyte, the battery undergoes charging.
This process occurs thousands of times during a battery's lifespan. The battery's power output and charging speed are contingent upon the rate at which this reaction takes place. Nevertheless, there is limited understanding regarding the precise mechanism of this reaction and the variables that influence its rate.
The model indicates that lithium intercalation is regulated by a mechanism called coupled ion-electron transfer, in which an electron is transferred to the electrode simultaneously with a lithium ion.
The researchers assert that insights from this model may inform the development of lithium-ion batteries that are both more efficient and capable of faster charging.
What we hope is enabled by this work is to get the reactions to be faster and more controlled, which can speed up charging and discharging.
Martin Bazant, Chevron Professor, Chemical Engineering, Massachusetts Institute of Technology
Martin Bazant is also a Professor of Mathematics at MIT.
The latest model could assist researchers in comprehending why modifying electrodes and electrolytes in specific ways enhances energy, power, and battery longevity — a procedure that has predominantly relied on trial and error.
This is one of these papers where now we began to unify the observations of reaction rates that we see with different materials and interfaces, in one theory of coupled electron and ion transfer for intercalation, building up previous work on reaction rates.
Yang Shao-Horn, J.R. East Professor, Engineering, Massachusetts Institute of Technology
Yang Shao-Horn is also a Mechanical Engineering, Materials Science and Engineering, and Chemistry Professor.
Shao-Horn and Bazant are the senior authors of the study. The lead authors include Yirui Zhang, PhD ’22, currently an assistant professor at Rice University; Dimitrios Fraggedakis, PhD ’21, now an assistant professor at Princeton University; Tao Gao, a former postdoctoral researcher at MIT who is now an assistant professor at the University of Utah; and Shakul Pathak, a graduate student at MIT.
Modeling Lithium Flow
For numerous decades, researchers have theorized that the speed of lithium intercalation at a lithium-ion battery electrode is influenced by the rate at which lithium ions can migrate from the electrolyte into the electrode. The team posited that this reaction was regulated by a framework referred to as the Butler-Volmer equation, which was initially formulated nearly a hundred years ago to characterize the rate of charge transfer in the context of an electrochemical reaction.
When researchers have attempted to assess lithium intercalation rates, the results they acquired were not consistently aligned with the rates forecasted by the Butler-Volmer equation. Additionally, achieving uniform measurements across various laboratories has proven challenging, as different research groups have reported measurements for the identical reaction that differed by a factor of as much as one billion.
The MIT team assessed lithium intercalation rates through an electrochemical method that entails the application of brief, repeated voltage bursts to an electrode. They produced these measurements for over 50 different combinations of electrolytes and electrodes, including lithium nickel manganese cobalt oxide, frequently utilized in electric vehicle batteries, as well as lithium cobalt oxide, which is present in the batteries that energize the majority of cell phones, laptops, and other portable electronic devices.
For these materials, the observed rates are significantly lower than those reported in the past, and they do not align with the predictions made by the conventional Butler-Volmer model.
The researchers utilized the data to formulate an alternative theory regarding the process of lithium intercalation at the surface of an electrode. This theory posits that an electron from the electrolyte solution must simultaneously be transferred to the electrode for a lithium ion to penetrate the electrode.
The electrochemical step is not lithium insertion, which you might think is the main thing, but it’s actually electron transfer to reduce the solid material that is hosting the lithium. Lithium is intercalated at the same time that the electron is transferred, and they facilitate one another.
Martin Bazant, Chevron Professor, Chemical Engineering, Massachusetts Institute of Technology
This coupled-electron ion transfer (CIET) reduces the energy barrier that must be surpassed for the intercalation reaction to occur, thereby increasing its likelihood. The mathematical framework of CIET enabled the researchers to predict reaction rates, which were confirmed by their experiments and were significantly different from those predicted by the Butler-Volmer model.
Faster Charging
In this research, the investigators demonstrated that adjusting intercalation rates by modifying the electrolyte composition is possible. For instance, substituting various anions can reduce the energy required for the transfer of lithium and electrons, thereby enhancing the efficiency of the process.
“Tuning the intercalation kinetics by changing electrolytes offers great opportunities to enhance the reaction rates, alter electrode designs, and therefore enhance the battery power and energy,” said Shao-Horn.
Shao-Horn’s laboratory and collaborators have employed automated experiments to create and evaluate thousands of electrolytes. These electrolytes are utilized in developing machine-learning models to predict electrolytes with improved functionalities.
The results may also assist researchers in designing batteries that charge more rapidly by accelerating the lithium intercalation reaction. Additionally, a further objective is to minimize the side reactions that can lead to battery degradation when electrons are extracted from the electrode and dissolve into the electrolyte.
“If you want to do that rationally, not just by trial and error, you need some kind of theoretical framework to know what are the important material parameters that you can play with. That’s what this study tries to provide,” said Bazant.
The study received financial support from Shell International Exploration and Production and the Toyota Research Institute via the D3BATT Center for Data-Driven Design of Rechargeable Batteries.
Journal Reference:
Zhang, Y., et al. (2025) Lithium-ion intercalation by coupled ion-electron transfer. Science. doi.org/10.1126/science.adq2541