Introduction
Polymeric composites with carbon black (CB) are very interesting materials because CB can be used as filler material and can importantly modify the electrical and mechanical properties of the used matrixes [1-3]. Modification and improvement of the mentioned properties are determined by the right selection of the couple polymer-CB. This has permitted a broad use of this kind of materials in multiple and numerous practical applications [1]. Conductive composites, for example, are used in electronic components, as antistatic shields against electromagnetic radiation [1] and they are good candidates for using them as solvent sensors [4-6].
Conductive material is randomly distributed into the dielectric phase (polymeric matrix) and the conductivity of composites can be controlled and modified in an extensive range of resistivities by varying the CB proportion [1]. Therefore for a good exploitation of these materials it is necessary to know the existent relationship between sample resistivity and the CB concentration for the particular choice of the polymer-CB pair. This can be experimentally achieved.
Resistivity versus CB percent plots are typically characterized by an abrupt transition wich separates the dielectric state from the conductive one [1, 7, 8]. The critical concentration that defines such transition is known as the percolation threshold. For polymeric composites, this critical concentration could vary from 4% CB (for poly methacrylic acid) to 34% CB (for SBR[1]). For polystyrene and polyethylene the threshold transition is 15 and 17% of CB respectively [1, 7].
The mechanism through which the electrical conduction occurs in polymeric systems is an unresolved problem in their totality. However percolation theory is one of the most used for explaining and modeling the electrical conduction in these systems. In this theory the electrical conductivity is developed through conductive networks build by the physical contact among CB individual particles which are dispersed into the polymeric matrix [8, 9]. In his context the percolation threshold corresponds to the lower CB concentration in which conductive CB chains take place. This percolation threshold depends on both preparation method and inherent characteristics of the polymer-CB pair [10-12]. The critical concentration value is a very important parameter for producing conductive composites. If the critical concentration is low, CB dispersion and distribution is easily achieved while high CB concentrations bring difficulties in the mixing process.
Traditionally for producing polymeric conductive composites an extended variety of oleo-polymers has been used [1-8]; however, an alternative is to use a polymeric matrix from natural and renovable sources as soybean oil, linseed oil, sunflower oil, etc. Polymers derived from those natural oils are taking importance in different areas [13] as engineering and aeronautic due to the fact that mechanical properties can be improved by reinforcing them with natural and synthetic fibers and clays, among others [14]. Recently electrical properties [15] were reported pointing that percolation concentration of carbon nanotubes was around 1%. So, polymers obtained from renewable sources are good candidates for being used in conductive polymeric composites.
Natural oils as soybean contain unsaturations that can be chemically modified through simple reactions. These reactions permit to introduce polymerizable groups [16, 17] as epoxy or acrylates which are available to give products with practical useful properties and characteristics [18, 19]. In this work we studied the effect of acrylated-epoxidized soybean oil (AESO) on the electrical properties of composites based on Poly(AESO-co-BMA) with CB. The idea of copolymerize AESO whit BMA was for improving the molding or extrusion processing properties [18, 20] and for modifying the electrical characteristics. The processing conditions were not satisfactorily achieved however the carbon black electrical percolation was only 1.2%CB. This concentration is much lower than traditional oleo polymers-based composites with CB.
Experimental Procedure
Materials
Acrylated-epoxidized soybean oil and butyl methacrylate were purchased from ALDRICH and they were used without any further treatment. THF (99.7%) used for dispersing the CB particles and benzoyl peroxide for polymerizing, were purchased from Merck without receiving additional treatments. Carbon black (CB) Vulcan XC72 (30 nm), was donated by Cabot Co.
Material preparation
To study the electrical properties two kinds of poly(AESO-co-BMA) + CB composites were synthesized. The first one was prepared from varying the monomer composition maintaining the CB proportion fixed. For the second kind the electrical properties were modified by changing the CB weight concentration for a particular monomer proportion. In order to compare the electrical behaviors of the composites PolyAESO, PolyBMA and copolymers using different monomer proportions without CB were synthesized. The general procedure to prepare all compounds consisted on obtaining a homogeneous mixture of monomers and CB before the polymerization. The mixing process was carried out by adding THF and shacking it using an ultrasonic processor UltrasonikTM 28X (50/60Hz) for 10 min. The temperature of ultrasonic bath was held below 8°C in order to avoid some initial polymerization. 0.05 ml of 0.5 M benzoyl peroxide was added and the reaction was carried out under nitrogen gas atmosphere. Finally the reaction tube was heated at 90°C by using a stable thermal oil bath. After the polymerization reaction finished (between 2 and 6 hr) the products were first washed in toluene, then in acetone and finally they were dried under vacuum for at least 48 hr. Five samples of each compound were prepared following the same procedure in order to verify the reproducibility of the results.
Electrical characterization
Current-voltage relationships were determined using a 6517A Keithley electrometer. Homopolymers, copolymers and composites were cut in 3 mm thick x 60 mm radius cylinder shape samples. Stannous paper sheets electrodes of 40 μm thick x 12 mm radius were prepared and the samples were placed between them. The DC applied voltages were varied from 1 to 50 volts in order to determine the current-voltage curves for each compound. CB percolation concentration was determined from the resistivity vs CB weight concentration curves using the phenomenological criteria.
Results and Discussion
Composites electrical properties at several CB%wt concentrations were evaluated for a broad monomers proportion range. Figure 1 shows the electrical copolymer and homopolymer resistivity variation as a function of monomers composition. Polybutyl methacrylate (polyBMA) and polyAESO registered at 1014 Ω cm and 1012 Ω cm resistivities, respectively. As AESO proportion increased (from 0 to30%wt) the electrical resistivity of copolymers decreased two orders of magnitude reaching a minimum. For AESO concentrations higher than 30% the resistivity has practically constant. We could explain this behavior in terms of the three dimensional cross linked structure of the copolymeric matrix imposed by the AESO monomer. After 30% of AESO no changes in the structure are produced by increasing the oil monomer concentration and then the electrical resistivity approaches the resistivity of pure polyAESO (Figure 1).
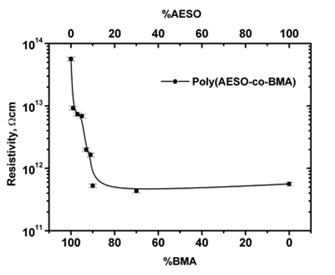
Figure 1: Resistivity variation of poly(AESO-co-BMA) + CB composites as a function of monomers proportion and reproducibility error bars (7%).
It is well known that electrical properties of composites are significantly influenced by the structural morphology of the host polymers. In agreement with our results, electrical properties of the poly(AESO-co-BMA)+CB can be modify by two different ways: the first consists in varying the monomers proportion maintaining the CB%wt constant and the second, way in varying CB%wt concentration maintaining constant the monomers proportion. In each case the electrical behavior is controlled by their own mechanisms. For the first the mechanism shows a continuous transformation from the dielectric to conductive state (Figure 2). In the second case the mechanism is explained by the percolation theory, as we can see in Figure 3. These behaviors have been observed for polystyrene:polyethylene blends [10]. In these systems there are well defined interphases between each polystyrene and polyethylene chains which permit a CB preferential distribution into the polymeric system. In our composites the structural array is three dimensional making easy the preferential electrical network paths among CB particles. The electrical properties of polymeric composites could be modified by varying the monomers proportion maintaining constant the CB concentration. In Figure 2 we show how the resistivity presents a sharp diminish as the AESO monomer weight percentage increased from 0 to10 at constant 10%wt. CB concentration. In this range of monomer compositions the electrical resisitivity changes seven orders of magnitude. In agreement with the phenomenological criterion, composites change from the dielectric state to the conductive regimen. These observations confirm that the cross linked matrix produces preferential CB conductive paths. The percentage of cross linked matrix in thermosetting polymers based on triglycerides has been measured [20]. In these materials the cross linking density depends on the acrylate functionality number; in our polymers this number could be associated to the AESO weight percent.
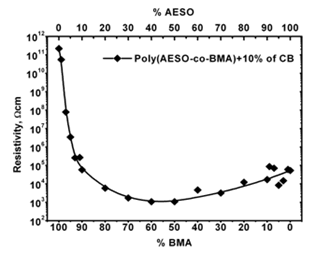
Figure 2: Electrical behavior for poly(AESO-co-BMA) composites as a function of monomers proportions for a constant CB concentration.
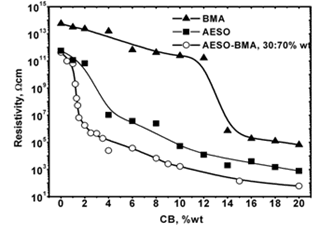
Figure 3: Polymeric matrix nature effect on electrical properties of composites respect to CB wt%.
The results from modifying the electrical resistivity by changing the CB concentration are shown in Figure 3. We can see the CB weight percent concentration effect on the electrical resistivity of polyAESO and polyBMA homopolymers-base composites and also 30:70%wt of poly(AESO-co-BMA) composite system. This last one was chosen based the results shown in Figure 1, since the electrical resistivity of copolymers (without CB) do not change for concentrations higher than 30% of AESO. For the systems shown in Figure 3 the results of the electrical behavior can be explained by percolation theory. The CB percolation threshold for these different systems was determined by the effective medium theory [21, 22]. In agreement with this theory, percolation XC corresponds to CB concentration which is reached when
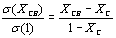
goes to zero. In this relation σ (XCB) is the composite conductivity which contains a XCB fraction of CB, and σ (1) is the conductivity of pure CB. Figure 4 shows the percolation threshold of the 30:70%wt of poly(AESO-co-BMA) + CB composite and it corresponds to 1.2% of CB. For the composites based on pure polyAESO + CB we obtained 4% and for the polyBMA-based composites it was around 14%. We can conclude that the morphology of the polymeric matrix could improve the electrical properties of composites materials.
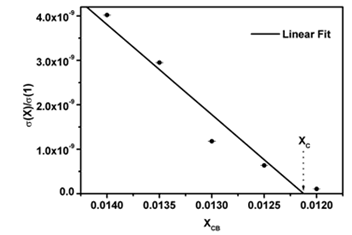
Figure 4: Percolation threshold estimated according to effective medium approximation.
Conclusions
We prepare in situ modified-composites soybean oil + CB based composites, with very low percolation concentration (1.2%). Composites electrical resistivity of poly(AESO-co-BMA) could be modified by changing the monomer or the CB concentrations. Both ways are strongly influenced by the chemical structure of polymeric matrix. Electrical resistivity through changing the monomer composition is controlled by a monotonic decrease until it reaches a minimum in which the electrical resistivity vary into the same magnitude order. This is in good agreement whit the proposal that the cross linked structures of the AESO:BMA reach a saturation level respect to ASEO proportion. When CB concentration is changed the electrical resistivity shows a typical behavior of ordinary conductive polymer composites, however electrical percolation threshold for the AESO:BMA system is reached a lower values than those reported for commercial oleo polymers based composites.
Acknowledgements
This work was supported by the Universidad Autónoma del Estado de México as projects No. 1806/2004A and 1981/2004B.
References
1. J. B Donnet, R. L Bansal and M-J. Wang, editors. “Carbon Black”, New York, (1993), Dekker and references there.
2. W. Brostow, M. Keselman, I. M. Harpaz, M. Narkis and R. Pierce, “Effects of Carbon Black on Tribology and Non-irradiated Ultrahigh Molecular Weight Polyethylene”, Polymer, Vol. 46, 5058-5064, 2005.
3. M. Knite, V. Teteris, B. Polyakov and D. Erts, “Electrical and Elastic Properties of Conductive Polymeric Nanocomposites on Macro- and Nanoscales”, Materials Science and Engineering C, Vol. 19, 15-19, 2002.
4. A. Márquez, J. Uribe and R. Cruz, “Conductivity Variation Induced by Solvent Swelling of an Elastomer-Carbon Black –Graphite Composite”, Journal of Applied Polymer Science, Vol. 66, 2221-2232, 1997.
5. A. Carrillo, I. R. Martín-Domínguez, D. Glossman and A. Márquez, “Study of the Effect of Solvent Induced Swelling on the Resistivity of Butadiene Based Elastomers Filled with Carbon Particles”, Part I. Elucidate second order effects”, Sensors and Actuators A, Vol. 119, 157-168, 2005.
6. M. C. Lonergan, E.J. Severin, B. J. Doleman, S. A. Beaber, R. H. Grubbs and N. S. Lewis, “Array-based Vapor Sensing using Chemically Sensitive, Carbon Black-Polymer Resisitors”, Chem. Maters, Vol. 8, 2298-2312, 1996.
7. R. San Juan-Farfán, S. Hernández-López, G. Martínez-Barrera, M. A. Camacho-López and E. Vigueras-Santiago, “Electrical Characterization of Polystyrene-Carbon Black Composites”, Phys. stat. sol., (c) 2 (2005) 3762-3765.
8. Z. Rubin, S. A. Sunshine, M. B. Heaney, I .Bloom and I. Balberg, “Critical Behavior of the Electrical Properties in a Tunneling-Percolation System”, Phys. Rev. B, Vol. 59, 12196-12199, 1999.
9. D. Stauffer and A. Aharony, “Introduction to Percolation”, London, Washington, DC (1992) Taylor and Francis. Chapters 1, 4 and 5.
10. F. Gubbels, S. Blacher, E. Vanlathem, R. Jerome, R. Deltour, F. Brouers and Ph. Teyssie, “Design of Electrical Conductive Composites: Key Role of the Morphology on the Electrical Properties of Carbon Black Filled Polymer Blends”, Macromolecules, Vol. 28, 1559-1566, 1995.
11. G. Beaucage, S. Rane, D. W. Schaefer, G. Long and D. Fisher, “Morphology of Polyethylene-Carbon Black Composites”, J. of Polymer Science: Part B. Polymer Physics, Vol. 37, (1999) 1105-1119.
12. J. F. Feller, D. Langevin and S. Marais, “Influence of the Processing Conditions on Sensitivity of Conductive Polymers Composites to Organic Solvent Vapors”, Synthetic Metals, Vol. 144, 81-88, 2004.
13. S. N. Khot, J. J. Lascala, E. Can, S. S. Moyre, G. I. Williams, G. R. Palmese, S. H. Kusefoglu and R. P. Wool, “Development and Application of Triglyceride-Based Polymers and Composites”, J. of Appl. Polym Scie., Vol. 82, 703-723, 2001.
14. R. Wool, S. Kusefoglu, G. Palmese, S. N. Khot and R. Zhao, “High Modulus Polymers and Composites from Plant Oils”, US Patent No. 6,121,398 (September 19, 2000).
15. M. In Het Panhuis, W. Thielemans, A. I. Minett, R. Leahy, B. Le Foulgoc, W. J. Blau and R. P. Wool, “A Composite from Soy Oil and Carbon Nanotubes”, International Journal of Nanoscience, Vol. 2, 185-194, 2003.
16. U. Biermann, W. Friedt, S. Lang, W. Lühs, G. Machmüller, J. O. Metzger, M. R. Klaas, H. J. Schäfer and M. P. Schneider, “New Synthesis with Oils and Fats as Renewable Materials for the Chemical Industry”, Agew. Chem. Int. Ed., 39 (2000) 2206-2224.
17. I. Hilker, D. Bothe, J. Prüss and H. –J. Warnecke, “Chemo-Enzymatic Epoxidation of Unsaturated Plants Oils”, Chemical Engineering Science, Vol. 56, 427-432, 2001.
18. J. La Scala, J. M. Sands, J. A. Orlicki, E. J. Robinette and G. R. Palmese, “Fatty Acid-based Monomers as Styrene Replacements for Liquid Molding Resins”, Polymer, Vol. 45, 7729-7737, 2004.
19. R. Wool, S. Küsefoglu, G. Palmese, S. Khot and R. Zhao, “High Modulus Polymers and Composites from Plant Oils”, US Patent No. 6,121,398 (September 19, 2000).
20. J. La Scala and R. P. Wool, “Property Analysis of Triglyceride-based Thermosets”, Polymer, Vol. 46, 61-69, 2005.
21. S. Kirkpatrick, “Percolation and Conduction”, Rev. Modern Phys., Vol. 45, 574-588, 1973.
22. R. Landauer, “The Electrical Resistence of Binary Metallic Mixtures”, J. of Applied Phys., 23 (1952) 779-784.
Contact Details
|