Innovative fibrous-shaped silica nanospheres, specified as KCC-1 (KAUST Catalysis Center),[1] have distinctive physical properties which have never before been noticed in silica materials. Professor J. M. Basset of King Abdullah University of Science and Technology (KAUST) developed these nanomaterials.
Properties of Silica Nanospheres
The nanospheres are formed by the arrangement of a fibrous surface morphology in a three-dimensional structure (Figure 1). In contrast to conventional pore-based silica, these nanospheres have a fibrous structure that increases accessibility to the available surface area, which, in turn, considerably increases the catalytic activity.
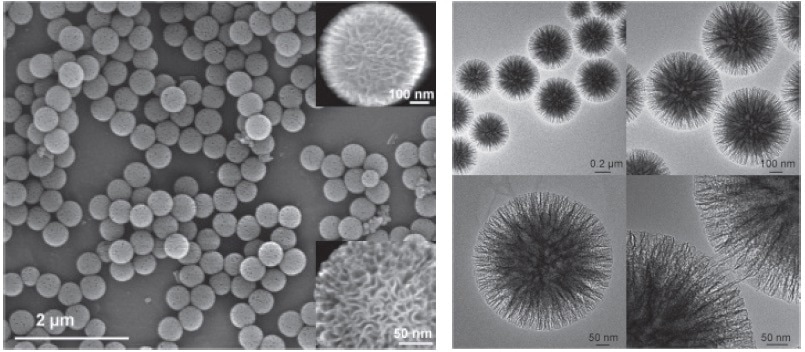
Figure 1. SEM and HRTEM images of silica nanospheres KCC-1
These materials demonstrate exceptional physical properties, including a fibrous surface morphology, high surface area, high mechanical stability, and excellent hydrothermal and thermal stabilities (Table 1). Even after a mechanical compression of up to 216 MPa pressure, the fibrous morphology of KCC-1 stays unaffected. The KCC-1 is excellent compared to the traditional MCM-41 type of silica, which is affected at the pressure of 86 MPa.[1]
| 96-5050 High Surface Area Silica Nanoparticles Kit Contains smallest unit size for each of the following |
| Product# |
Category |
Grade |
Particle Size
(nm) |
Surface Area
(m2/g) |
Pore Volume
(cm3/g) |
| 14-6100 |
Large |
(KCC-1 L1) |
~900-1000 |
~700 |
~1.4 |
| 14-6110 |
Large |
(KCC-1 L2) |
~900-1000 |
~600 |
~1.2 |
| 14-6120 |
Large |
(KCC-1 L3) |
~900-1000 |
~550 |
~0.9 |
| 14-6200 |
Medium |
(KCC-1 M1) |
~400-450 |
~400 |
~0.7 |
| 14-6210 |
Medium |
(KCC-1 M2) |
~300-350 |
~600 |
~0.6 |
| 14-6300 |
Small |
(KCC-1 S1) |
~130-190 |
~380 |
~0.8 |
| 14-6310 |
Small |
(KCC-1 S2) |
~40-50 nm |
~520 |
~1.3 |
Components also available for individual sale
Summary
A variety of heterogeneous catalysts, produced using KCC-1 as a supporting material, have been exhibiting good catalytic activity for several transformations of research and industrial significance. As a catalyst support, carrier or sorbent, KCC-1 has the potential to exhibit excellent activity in comparison with common mesoporous silica materials in energy-related processes[2-3], different organic reactions[4-7], biomedical applications and drug delivery systems[8], optoelectronic devices[9], and many more.
References and Further Reading
- Angew. Chem. Int. Ed. 2010, 49, 9652.
- Chem. Mater. 2015, 27, 8237.
- ACS Catalysis. 2016, 6, 2770.
- ChemSusChem 2012, 5, 85.
- Green Chem., 2016, 18, 5890.
- Angew. Chem. Int. Ed. 2011, 50, 2747.
- RSC Adv., 2017, 7, 24885.
- Langmuir 2014, 30, 10886.
- J. Mater. Chem. B, 2015, 3, 3201.

This information has been sourced, reviewed and adapted from materials provided by Strem Chemicals.
For more information on this source, please visit Strem Chemicals.