PVDF (Polyvinylidene difluoride) is a well-known fluoropolymer (Figure 1). PVDF was developed from PTFE and, as a result, exhibits similar behavior and is also used as a coating. PVDF was patented in 1948 and the rights to produce the material have changed hands several times since then. PVDF is marketed as Hylar® (Solvay), Kynar® (Arkema) to name just two examples.1
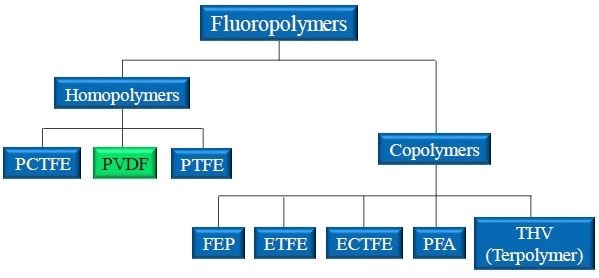
Figure 1. Landscape of popular fluoropolymers. PVDF is a homopolymer produced from the joining of identical monomer units. PVDF is closely related to PCTFE and PTFE.
PVDF has some unique characteristics that set it apart from other fluoropolymers, with one of these being its multiple conformations. These differences make PVDF easier to manipulate in both pre- and post-processing, meaning it can be used in a wide range of applications; including applications where PTFE cannot be used.
Since it was commercialized the application areas of PVDF have become increasingly wide and the material is used in many unexpected industries. For this reason, PVDF currently is the second biggest fluoropolymer in terms of marketing share.
Discovery, Synthesis and Structure
PVDF synthesis followed the first synthesis of PTFE in the late 1930s at DuPoint.2 In a similar fashion to PTFE synthesis, PVDF is produced via a radical polymerization of monomers of vinylidene difluoride (Figure 2). Even though PVDF has alternating -CH2 and -CF2 groups it is still a homopolymer because it consists of just one repeating monomer unit.
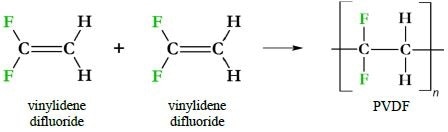
Figure 2. PVDF immediate precursors and synthesis. One canonical means of synthesizing PVDF is via a radical reaction joining vinylidene difluoride monomers.
As PVDF is simple to synthesize it is easy to wrongly believe that it is a simple polymer. PDVF is one of the only fluoropolymers that can adopt several different stable configurations in its crystalline state. Whilst PVDF does show some similar behavior to PTFE it is this conformational diversity that makes it different to other fluoropolymers.
The fluorine atoms in PVDF are responsible for the different conformations that it can adopt. The fluorine atoms in PVDF each possess three lone pair (six non-bonded) electrons which means they have a high electron density; and this high charge density results in the atoms repulsing each other.
The repulsion between the fluorine atoms in PVDF result in (at least) four different stable conformations: form I (β), form II (α), form III (γ), and form IV (δ). PVDF also has amorphous phase conformations though, as they are disordered, they are undefined.
Form I of PVDF has a canonical zig-zag conformation, similar to the conformation of a linear, unbranched hydrocarbon chain (Figure 3a). Form II of PVDF (PVDF α) is the form most commonly seen at room temperature due to its higher stability (Figure 3b). The enhanced stability of form II is the result of vicinal (i.e. neighboring) pairs of fluorine atoms being held further apart than they are in form I.
[The following information will generally be concerning form I (for illustration) and form II.]
![Structure of PVDF ß (form I) and a (form II). A) Form I (ß) is a typical zig-zag conformation similar to simple saturated hydrocarbon chains. B) Form II (a) adopts an up-and-down or undulating conformation (as shown in 2-D) and positions the fluoride atoms at a greater distance from one another than in form I. PVDF a is more stable than ß making PVDF a the most common form occurring spontaneously and at room temperature. [For A and B, illustrations are: stereo bond-line drawing (upper), ball-and-stick model (middle), and space-filling model (lower). Conformations of PVDF are variable in melt and amorphous phases].](https://www.azom.com/images/Article_Images/ImageForArticle_17670_450145530209837963353.jpg)
Figure 3. Structure of PVDF β (form I) and α (form II). A) Form I (β) is a typical zig-zag conformation similar to simple saturated hydrocarbon chains. B) Form II (α) adopts an up-and-down or undulating conformation (as shown in 2-D) and positions the fluoride atoms at a greater distance from one another than in form I. PVDF α is more stable than β making PVDF α the most common form occurring spontaneously and at room temperature. [For A and B, illustrations are: stereo bond-line drawing (upper), ball-and-stick model (middle), and space-filling model (lower). Conformations of PVDF are variable in melt and amorphous phases].
Processing
One factor that makes PVDF more suitable for commercialization than PTFE is that it is meltprocessable unlike PTFE. Including the alternating –CH2 groups of PVDF rather than all –CF2 groups as PTFE reduces the melting point to ~170 °C (338 °F) for PVDF compared to 327 °C (621 °F) for PTFE. (PVDF melting point may nevertheless vary due to the wide assortment of available PVDF resin grades). Processing of PVDF is easy and can be carried out with most industry-standard equipment.3
Processing can usually take place without having to use lubricants or plasticizers, allowing pure PVDF to be synthesized.4 Significant venting must take place during the final stages of injection molding with PVDF to prevent burning or “dieseling”.3 PVDF is amenable to all common manufacturing processes including extrusion, compression molding, injection and blow molding, and impregnation and coating applications (Table 1).
Table 1. PVDF processing suitability. PVDF is easily processed with industry-standard equipment and is amenable to most processing and production methods.
| Processing Method |
Suitability |
| Injection molding |
Yes |
| Extrusion (profiles, films, sheet, tubing, and coating) |
Yes |
| Blow molding (thin films) |
Yes |
| Compression molding |
Yes |
| Impregnation and coating |
Yes |
When injection molding with PVDF the use of surfaces plated in chrome or nickel is preferred as it helps to prevent pitting of the surface.3 The easy processability and high adaptability of PVDF is one of many characteristics that make PVDF a popular industrial material.
PVDF Properties
Physical and Mechanical
PVDF possesses a combination of properties that puts it on a par with some of the best-performing fluoropolymers. PVDF tends to form long polymer chains, meaning it has a high molecular weight. Regular PVDF products are of light weight and have a color that ranges between translucent to white (Table 2). PVDF is lighter than perfluorinated polymers, such as fluorinated ethylene propylene (FEP) or PTFE, but heavier than polyether ether ketone (PEEK).
Table 2. PVDF typical physical properties. PVDF chemical structure gives it many exceptional properties including chemical resistance and biocompatibility. For sterilization, autoclave and ETO are preferred over gamma irradiation. (Methods are ASTM test standards except where indicated).
| Property |
ASTM |
Value (natural polymer) |
| Appearance |
– |
Whitish to translucent |
| Density (g/cm3) |
D792 |
1.76 - 1.82 |
| Specific Gravity (23 °C) |
D792 |
1.76 – 1.82 |
| Water Absorption (50% rh; %) |
D570 / ISO 62-1 |
0.02 - 0.07 |
| Refraction Index |
D542 |
1.41 |
| Limiting Oxygen Index (LOI) 3.2 mm thickness |
D2863 |
44 |
| Biocompatible |
USP Class VI |
Yes |
| Chemical Resistance |
– |
Excellent |
| Sterilization |
– |
Autoclave, ETO, and gamma |
PVDF contains strong and short C-F and C-H bonds which are very unreactive, making PVDF highly chemical resistant (even at high temperatures) and an extremely low water absorber. This resistivity means that it is possible to steam clean PVDF at 140 °C (284 °F) for autoclaving, and it can also be cleaned using ETO or gamma sterilization, with the former method being preferred.3
The inertness of PVDF means it is biocompatible, classified as a USP Class VI plastic. The biocompatibility of PVDF means it is used extensively in the biomedical and medical industries as well as its established industrial uses. In addition, the different conformations of PVDF, each with their own properties, mean that PVDF has some unique applications. As it is easy to process PVDF can also be a popular material for applications even where its performance is not perfect.
PVDF’s mechanical properties also demonstrate why it is used in so many different applications. PVDF has a semi-crystalline structure of around 50% crystallinity. The polymer shows strong dimensional stability, though highly crystalline PVDF, of close to 70% crystallinity, can show shrinkage and this should be considered if products with critical dimensions are required.3 In addition to this highly crystalline PVDF can also grow voids during the process of molding.3
When compared to other fluoropolymers PVDF has a high resistance to abrasion and hardness, whilst also being relatively rigid (Table 3). It is mechanically strong and also shows a tensile strength similar to that of PEEK; which is useful for manufacturers who do not want to use a polymer with aromatic groups.
Table 3. Typical PVDF mechanical properties. PVDF possesses excellent mechanical properties even at the upper range of its working temperature. PVDF also performs well under load. (Methods are ASTM test standards except as indicated).
| Property |
ASTM |
Value (natural polymer) |
| Tensile Strength (MPa) |
D638 |
14 - 55 |
| Elongation at Break (%) |
D638 |
20 – 800 |
| Modulus of Elasticity (GPa) |
D638 |
1.3 – 2.2 |
| Flexural Modulus (GPa) |
D790 |
1.38 – 2.31 |
| Flexural Strength (MPa) |
ISO 178 / D790 |
37 – 80 |
| Hardness (Shore D) |
D2240 |
50 – 80 |
Impact Strength (23 °C; kJ/m2)
notched
unnotched |
ISO 180 |
8
no break |
| Coefficient of Friction |
D1894 |
0.14 – 0.54 |
PVDF works well at bearing loads with less creep than the majority of other melt-processable plastics. PVDF’s friction coefficient is relatively low, being lower than the majority of fluoropolymers, though it is higher than PTFE – which is the most popular, low-friction fluoropolymer.
The physical properties of PVDF are only weakly impacted following long-term exposure to UV meaning it is a useful material for outdoor applications. PVDF’s high mechanical toughness and strength, alongside its crystalline and dimensional stability, means it is classed as one of the best performing fluoropolymers.
Thermal
PVDF shows good thermal performance over its application temperature range. PVDF has an adequate thermal stability, even at temperatures below zero, with a moderate maximum of 150 °C (302 °F), (Table 4). Even molten PVDF at 250 °C (482 °F) does not exhibit weight loss.
Table 4. PVDF thermal properties. While PVDF’s upper service temperature may appear comparably moderate, it retains many desirable mechanical properties at these temperatures. PVDF’s resistance to burning is also a highly preferred trait. (Methods are ASTM test standards except where indicated).
| Property |
Method |
Value (natural polymer) |
| Thermal Conductivity (W/m-K) |
D433/ISO 22007-4/C-177 |
0.14 – 0.20 |
| Maximum Service Temperature (°C) |
UL 746 |
150 |
| Minimum Service Temperature (°C) |
UL 746 |
-40 |
| Melting Point (°C) |
D4591/D3418/ISO 12086/DOW Method |
117 – 172 |
| Glass Transition Temperature (°C) |
E1356 (DSC) or E1545 (mechanical) |
-48 to -38 |
| Decomposition Temperature (°C) |
E1131 |
375 |
| Coefficient of Thermal Expansion, Linear (µm/m-°C) |
D696 |
80 – 194 |
| Flammability Rating (UL 94) |
D2863 |
V-0 |
The low thermal conductivity of PVDF makes it a popular material for applications that require a heat barrier. In addition, PVDF is not very combustible and requires an atmosphere of at least 40% oxygen to burn, which is 19% more oxygen than is present in ambient conditions.
The low thermal conductivity of PVDF means it finds application as a heat barrier. It is also very difficult to burn, requiring an atmosphere of at least 40% oxygen (compared to the 21% concentration of oxygen in ambient conditions) to combust in a sustained fashion. PVDF is UL 94 V-0 rated in terms of flammability and is low smoke (i.e. self-extinguishing), which is of benefit in critical applications such as automotive and aerospace.
This behavior makes PVDF a plastic of choice for use in sustained, high-temperature applications.
Electrical
PVDF is a moderate insulator (Table 5). The α form of PVDF is the only non-polar conformation so does not show piezoelectric or pyroelectric behavior, this makes it more insulating than its other forms (β, γ, and δ).5,6 The majority of synthesized PVDF will contain some amorphous regions which result in reduced insulation in the α form.
Table 5. PVDF electrical properties. PVDF possesses moderate dielectric traits compared to perfluorinated polymers. PVDF dielectrics, however, can be influenced during processing to alter dielectric properties. (Methods are ASTM except where indicated).
| Property |
ASTM |
Value (natural polymer) |
| Dielectric Constant (1 MHz) |
D150 |
4.5 – 13.5 |
| Dielectric Strength (V/mil) |
D149/IEC 60243-1 |
254 – 1100 |
| Volume Resistivity (Ω–cm) |
D257/IEC 60096 |
2 × 1014 |
Weakly insulating PVDF can be preferable for use in applications that require static dissipation. PVDF’s highly variable insulating behavior is demonstrated in its wide breakdown voltage (the voltage at which material failure occurs) and dielectric strength ranges. This means that manufacturers should determine what type of PVDF is most appropriate for their application prior to purchase.
Finishing
PVDF can be produced in nearly any form that is amenable to the processes described in Table 1, with just some examples being sheets, rods, tubes, drawn fibers and monofilaments, cast parts and membranes. The ease of machining and finishing of PVDF; with PVDF machined in a similar fashion to polyamides, is another advantage of working with PVDF.
When working with PVDF it is best practice to leave unfinished PVDF products to equilibrate overnight to its external environment prior to fine machining. Some processes such as turning and milling should be carried out carefully as they can result in melting of the chip.
In such instances a cooling fluid can be used, with many different options available thanks to PVDF’s chemical inertness. It is also advised to carry out rough reaming and pre-drilling before finishing takes place.
To help in the machining process, many manufacturers provide guidelines to address critical issues and to help achieve precision parts fashioned from PVDF.
Applications
PVDF can be used in a wide range of applications. It’s properties (namely chemical resistance, heat tolerance, toughness and strength (Table 6) are favorable to many industrial applications.
Table 6. Survey of PVDF applications. PVDF’s key properties such as mechanical strength and toughness and its purity and chemical resistance are highly preferred over a broad spectrum of industries. PVDF’s ease of processing gives it additional advantages in these areas over other comparable polymers.
| Application or Industry |
Key Benefits |
| Aerospace and automotive |
UL 94 V-0 flammability rating, low smoke |
| Chemical manufacturing |
Chemical, heat resistance; toughness |
| Energy (oil and gas) |
Chemical resistance |
| Exterior (outdoors) |
Protective UV coating or paint additive to increase product longevity |
| Fluid handling |
Chemical resistance, purity |
| Pharmaceutical, food processing |
Biocompatibility; chemical and heat resistance; mechanical performance under load |
| General industrial |
Toughness, easily machined |
The high purity and strong chemical resistivity of PVDF means it is a popular choice for the coating of chemical transport systems and PVDF is frequently found in chemical plants in the form of fittings and valves. For the same reasons, as well as the high abrasion resistance and toughness of PVDF products, PVDF also tends to be present as flanges, pump components and spacers.
Some grades of PVDF have been approved by the USDA or FDA for use in applications where it will be in contact with food. PVDF is a favorable material for the production of food trays as it can be sterilized at high heats without the shape of the tray being impacted. For a similar reason PVDF is also popular in the pharmaceutical industry as it can easily be autoclaved; whilst also offering a high chemical resistance and a good load carrying ability.
PVDF is favorable for applications that involve high levels of mechanical stresses and temperatures. The dielectric properties of PVDF also make it useful when static dissipation is required. In contrast to this if PDVF has been synthesized to show insulating properties it is useful as a coating for wiring for use in high-temperature, critical applications such as in the aerospace sector. PVDF is highly suited to aerospace, and the entire vehicle industry, as it is inert to burning with a flammability rating of V-0.
The stability of PVDF towards UV radiation means it is used in many outdoor applications, such as a paint additive or protective coating to improve outdoor lifetime.
The key characteristics of PVDF, namely its high mechanical toughness, with respect to temperatures and loads, and chemical inertness, make it a highly versatile material and the second most popular fluoropolymer available.
Summary
PVDF polymers possess a strong mixture of different desirable properties whilst also being easy to process; with the presence of alternating -CH2 groups making the polymer melt processable at lower temperatures. The four identified stable crystalline forms of PVDF, α, β, γ and δ, make it a unique fluoropolymer.
α-PVDF is its most stable configuration making it the most prevalent at ambient temperatures, this is also the only configuration that exhibits no piezoelectric or pyroelectric behavior.
PVDF
PVDF is relatively unique in that it keeps many of its favorable characteristics even at the high end of its service temperature. PVDF is highly chemically inert and has a high tensile strength, which it retains under high thermal and mechanical stresses.
PVDF is not very combustible making it a desirable material for use in critical applications such as aerospace. The inertness of PVDF makes it particularly biocompatible, being used in medical devices and with some grades used in food packaging. The high purity of PVDF means it is a polymer of choice in the pharmaceutical and chemical industries.
Whilst it is not the best insulator PVDF is often used in applications that require static dissipation. The polymer can be formed into shapes such as membranes, tubing, films and more; all using regular, non-specialized plastic machining systems and protocols.
Whilst PVDF is not one of the best performing fluoropolymers its ease of manufacturing and versatile nature mean it is nonetheless highly useful and highly popular.
Table 7. PVDF advantages and limitations. PVDF provides a balance of properties allowing it to compete with other more costly polymers. PVDF also shows exceptional long-term UV (outdoor) performance.
| Advantages / Benefits (+) |
Limitations (–) |
- Ease of processing (melt processable)
- Mechanical strength
- Retention of mechanical properties
- Chemical and dimensional stability
- Excellent chemical resistance
- Low weight
- Excellent UV resistance
|
- Incompatible with glass fiber
- Cost
- Moderate upper service temperature
- Moderate insulating capability
|

This information has been sourced, reviewed and adapted from materials provided by Zeus.
For more information on this source, please visit Zeus.