Life Expectancy of Current Cemented Implants Depending on a patient’s mobility, weight, age, and other factors, around 15-25% of hip replacements, most of which use cemented implants - fail at around 15 years. In elderly, inactive patients the cemented implant is essentially good enough, but currently there is no suitable procedure for young (below 55 years) active patients, particularly males who are at a higher risk of requiring revision surgery to fit new implants when the old ones fail. Porous Coating to Extend Implant Life The answer could lie in a new porous coating developed at Oxford University aimed at greatly extending the working life of an implant by encouraging bone growth, not only to keep the elderly happy for the rest of their lives, but also to benefit patients in their 50’s or younger. Beyond hip implants, the coating’s ability to carry biological/bioactive materials could have future implications for cartilage growth or drug delivery. Implant Statistics and Causes In the UK, more than 50,000 artificial hip replacement operations are carried out each year, mainly because of osteoarthritis, with 1% of the population benefiting from the surgery. ‘Implants are mainly successful in patients aged 55 or older,’ says Peter Wilshaw from the Department of Materials at the University of Oxford, and one of the inventors behind the development of the new coating. ‘The problem comes when you are faced with younger patients.’ Currently Available Cementless Coatings and Their Shortcomings In recent years, cementless coatings have been developed to promote bone growth on the surface of implants, but these methods are expensive, and the coatings are often brittle. Based on hydroxyapatite (HA) and calcium phosphate, the coating is deposited on the implant using high-temperature plasma spray technology. ‘There is some success with these,’ says Wilshaw, ‘but it is by no means clear that they will actually, in the long-term, be the most applicable coating for implants.’ Using this approach it is difficult to control the stoichiometry, porosity, pore shape and topography of the coating. Furthermore, it is also difficult to make it stick, which can cause it to break off in the body. If this happens, the particles can get between the articulating surfaces, i.e. the ball and socket, leading to third-body wear. This dramatically speeds up the wear of these parts and can also damage the surrounding bone, which leads to aseptic loosening and ultimately the implant fails. A New Coating Approach The invention made by Wilshaw and his colleague Eva Palsgard, from the Centre for Surface Biotechnology at the University of Uppsala, Sweden, consists of a process for forming a porous inorganic coating on suitably prepared metals, ceramics, plastics and composites, the pores of which can be loaded with bioactive materials in order to promote bone, or other tissue growth. ‘Our approach was to look for a new coating that was not hydroxyapatite-based,’ says Wilshaw. ‘We have gone totally away from this approach by using a different porous ceramic material, in particular one that can be easily produced at room temperature to create a robust and corrosion resistant coating using standard commercial processes.’ The anodised aluminium coating has been known for many years but never before applied to the problem of implants for use in hips or other joints. Limitations of Hydroxyapatite Coatings ‘What you want from an ideal coating is for bone to grow right up to the implant and bond to it. The traditional approach used at the moment involves coating the implant with hydroxyapatite, which as far as the bone is concerned is a good thing,’ says Wilshaw. However, as he explains, it is not ideal. Not only are there problems with making the hydroxyapatite stick, but also the effect of hydroxyapatite on the growth of bone is not as vigorous as some other materials. Hydroxyapatite is osteoconductive, which induces the bone cells to attach and move along the surface of the implant, but ideally the implant surface should be osteoinductive and have properties that promote the generation of new bone material, and so lock the implant firmly in place within the hip. Increasing the Osteoconductivity of the Implant The porosity of the Oxford coating offers a means of loading the pores with osteoconductive, bioactive material, such as Bioglass. Bone cell growth and proliferation on the surface of the implant is promoted, resulting in a significantly stronger bond to the parent tissue. Consequently, the rate of implant failure would be significantly reduced and the service lifetime of implants increased. ‘We needed a material that would fit into the pores, and our pores are rather small (0.2 microns in diameter) says Wilshaw. ‘Bioglass comes in sol form allowing us to get the small particles into the pores. For hip implants you want them to last a very long time, ideally the life of the patient. We weren’t sure we could use a biological/organic system that would give that longevity.’ The Patented Porous Coating Process Patented by Isis Innovation, Oxford University’s technology transfer company, the porous inorganic coating is formed by depositing aluminium layers of one to two microns thick, onto the titanium alloy implant using electron beam evaporation, or other inexpensive, commercial processes. The aluminium layers are then anodised to produce a surface layer of anodised aluminium oxide (AAO). The aluminium/oxide boundary moves inwards as the process continues. The surface oxide contains roughly parallel pores extending perpendicular to the surface. The pore diameter depends on the anodising voltage used, while pore length is dependent on the anodisation time. Voltages of ~10-160V will give pore diameters of ~10-160nm, which can be widened by chemical dissolution. Bioglass, or other bioactive materials, can then be inserted into the pores. ‘Aluminium was chosen because it is the only material that produces this scale of pore structure when anodised,’ says Wilshaw. | 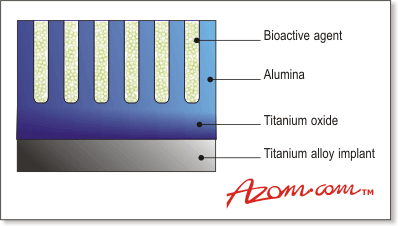
| | Figure 1. Schematic of the coating showing anodised aluminium coated titanium alloy with bioactive material in the pores. | ‘Other people have done anodisation of titanium and tantalum, but none of them produce this structure of pores,’ says Robert Adams Project Manager at Isis Innovation. Conversion of Aluminium to Aluminium Oxide ‘One of the things that people are slightly worried about is aluminium being left embedded in the anodised aluminium,’ says Wilshaw. ‘In all the tests we have done, we haven’t seen any signs of aluminium metal remaining, so we are pretty sure that this process actually converts all of it to anodised aluminium oxide.’ Bonding Between Implant and Porous Layer In the laboratory, the coating has undergone a range of sheer and tensile tests, and measurements have been carried out on mirrored polished surfaces. ‘This is the worst kind of surface in terms of measuring bond strengths,’ says Wilshaw. ‘Because we have taken the worst case scenario, our results are very conservative.’ It has been difficult to measure the actual strength of the bond between the implant and the porous layer because the glue used in the experiments has always failed first at 20MPa - Wilshaw expects even better performance on a rough surface. ‘This is already a good result,’ he says. As far as we have found, data for hydroxyapatite on a polished surface hasn’t produced results as good as these.’ Biological Behaviour of the Porous Coated Materials At the moment, there is an issue surrounding cementless implants relating to how long a patient has to be immobile after the operation. With cemented implants, a patient can be mobile fairly quickly because there is already some sort of bond. With cementless implants a patient has to wait for the bone to grow to the implant, which means they are in hospital for longer periods. Biocompatibility tests carried out by Marjam Karlsson from the University of Uppsala, under the guidance of Lucy Di Silvio from the Royal National Orthopaedic Hospital, Middlesex, UK, have shown some promising results for bone growth. Success to Date As a control, the researchers used a material designed to promote cell growth called Thermonox. In comparison, without the bioactive material, the coating actually matched or performed slightly better than the tissue culture plastic. ‘This was a surprise to us and we are currently trying to work out why this coating works as well as it does,’ says Wilshaw. ‘It may be as a result of the very fine pore structure we have interacting in some way, either with proteins coming from the body fluids, or with the cells themselves. It could also be phosphate present in the surface layer that the bone cells are responding to - we don’t know yet.’ At the moment, the researchers are not in a position to say that their porous coating is a bioactive material, and further work in this area is envisaged. Stability of the Porous Coating In addition, to see how quickly the porous material would dissolve, a series of cell culture tests were carried out using actual bone cells in a stimulated environment similar to the body. ‘We found the dissolution rate of the aluminium oxide was in fact, as you would expect, incredibly slow,’ says Wilshaw. ‘Even in that aggressive environment, you would only expect to get 10% loss of the coating in 10 years.’ Wilshaw is very encouraged by these results and says an implant coated with this porous material should last at least 30 years. Increasing the Implant Life Time Further increases in implant life time are possible by the use of ‘slow release’ bioactive compounds, which may be loaded into the pores of the coating. ‘Because the pores are long and thin we could put material at different depths,’ says Wilshaw, ‘so that the material that is seen the moment the operation is completed is the stuff that dissolves rather quickly and kick-starts bone growth to give you the bond. This is the material that is osteoconductive. Once this is used up, you then get a slow release of other materials that keeps the implant in place.’ Loading the Pores of the Coating with Osteoconductive Bioglass The technology was only launched in its current form last year. The next step is actually loading the pores with Bioglass and getting a commercial partner on board to enable in vivo studies to be carried out. The results achieved so far have been extremely promising and the researchers expect that when they load the pores with Bioglass the results will be even better. It is possible that future patients with implants coated with this material could be mobile in a much shorter period of time. Other Applications for the Porous Coated Implants Although Wilshaw and his team have been concentrating on developing the coating for hip implants, it could be used to coat anything in which you need a metal strongly bonded to bone, such as knees and other joints. One application that could prove less of a mountain to climb is dental implants, because they are easier to introduce into medical practice. In the longer term, and perhaps a more speculative idea, is using the coating to encourage cartilage growth. ‘We have a material that is actually a host for different agents,’ says Wilshaw. Modifications on the Existing Technology ‘In the future one of the things we would like to do is see whether we can impregnate our pores with a material that stimulates cartilage growth. Instead of loading the pores with Bioglass to stimulate bone growth, you could load them with a protein to stimulate cartilage growth.’ Potentially, the porous coating could be used to deliver drugs locally within the body, in which you would tailor the contents of the pores for the particular application. Wilshaw and his team have already been contacted by a biomaterials company interested in putting antibiotic drugs into the pores to prevent infection. Plans for Commercialisation In the short-term, in vivo studies are needed to convince the medical industry of its viability. ‘I want to see it in people improving their quality of life, which means it has to be commercialised,’ says Wilshaw. ‘Furthermore, it seems if people take on the commercial risk, they will want to see in vivo results. Therefore, logic demands that if it is going to end up in humans improving their quality of life, then in vivo measurements have to be done at some stage.’ Isis Innovation believes that, realistically, if a company takes the technology on and starts doing in vivo studies this year, then implants could appear in the marketplace within five years. Summary This new porous coating shows great promise for future implants and potentially drug delivery systems, with a view to increasing the success rate of hip implants in particular, and improving the longevity of replacement surgery It certainly has the potential to open a door for a category of people for whom, at the moment, hip implants are not surviving long enough. |