Quantitative ethylene oxide analysis in Polysorbate 80 excipient is greatly simplified using SIFT-MS, with a time to first test result that is eight-fold faster than the current compendial method and a daily sample throughput that is 9- to 14-fold higher.
Image Credit: ShutterStock/Irina Anosova
Ethylene oxide (EtO) is used extensively as a feedstock in the chemical industry,1 including in the manufacture of polyethylene glycols (PEGs), which are used as surfactants and emulsifiers in cosmetics, perfumes, and pharmaceuticals.
It also finds wide application as a sterilant in the pharmaceutical and medical implants industries due to its bactericidal, sporicidal, and virucidal properties while demonstrating compatibility with a broad array of biomaterials with which radiation and heat sterilization are otherwise incompatible.2
However, this toxicity is not confined to microbes: it poses a mutagenic risk to humans.3 Resultingly, it is crucial to detect trace ethylene oxide impurities.
Polysorbate 80 is a polyethylene oxide available under a variety of trade names,4 including Tween 80®, and is typically used as an emulsifier in a range of pharmaceutical products. The United States Pharmacopeia (USP) monograph Polysorbate 80 outlines the traditional gas chromatography-flame ionization detection (GC-FID) method for EtO analysis.5
This approach is labored from the perspective of sample preparation as well as performing sample analysis.6 The rate-limiting step in sample preparation is the 6-hour purification of the PEG matrix corresponding to the sample (1 mL of Polysorbate 80 in 1 mL of N, N-dimethylacetamide, and 0.2 mL of water).
Furthermore, the GC-FID cycle run time is 38 minutes per sample.
In this article, a streamlined alternative analytical method is discussed. By taking advantage of the greater sensitivity headspace analysis when coupled with selected ion flow tube mass spectrometry (SIFT-MS), a significantly lower amount of Polysorbate 80 is required when compared to headspace GC-FID.
This reduction eliminates the need for matrix matching and therefore removes the necessity to run the 6-hour PEG purification step.
Moreover, headspace-SIFT-MS analysis of ethylene oxide in Polysorbate 80 offers a nine-fold greater sample throughput than GC-FID and delivers a faster time to first test result when calibrations, blanks, and a system suitability test (SST) are taken into account (85 minutes compared to 6 hours; see Figure 1).
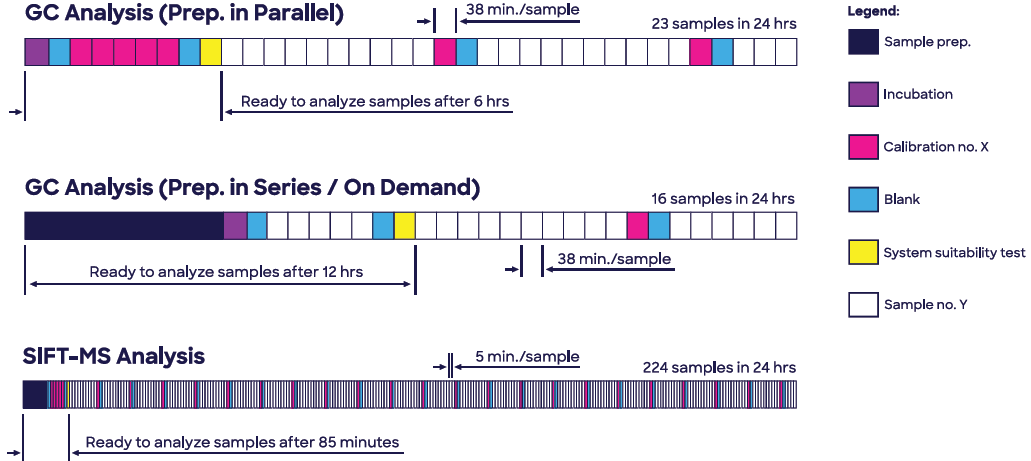
Figure 1. Headspace SIFT-MS enables analysis of ethylene oxide in Polysorbate 80 (Tween 80®) to be conducted at significantly higher throughput, while eliminating very slow sample preparation due to enhanced sensitivity. Image Credit: Syft Technologies
Method: The SIFT-MS Technique
For this application, a Syft Technologies Voice200ultra SIFT-MS instrument operating on helium carrier gas was used. SIFT-MS (Figure 2) utilizes soft chemical ionization (CI) to produce mass-selected reagent ions that can react rapidly with and quantify VOCs close to part-per-trillion concentrations (by volume, pptV).7
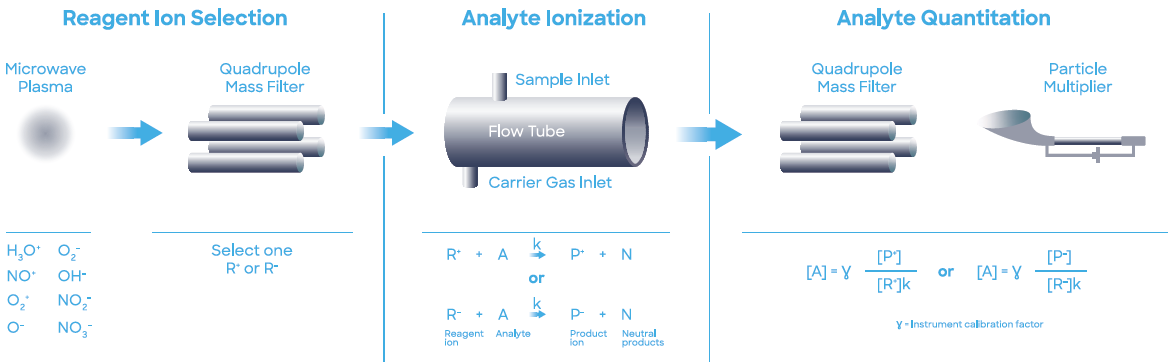
Figure 2. Schematic diagram of SIFT-MS – a direct, chemical-ionization analytical technique. Image Credit: Syft Technologies
Up to eight reagent ions (H3O+, NO+, O2+, O-, OH-, O2-, NO2- and NO3-) acquired from a microwave discharge in air are now used in commercially available SIFT-MS instruments.8 These reagent ions respond to VOCs and other trace analytes in well-controlled ion-molecule reactions, but they do not react with the main components of air (N2, O2, and Ar).
This facilitates the direct analysis of air samples in real-time at trace and ultra-trace levels without pre-concentration. Rapid switching between reagent ions offers greater selectivity because the multiple reaction mechanisms offer measurements of each analyte.
The multiple reagent ions repeatedly eliminate uncertainty from isobaric overlaps in mixtures that are comprised of multiple analytes.
Automated MHE analysis was performed using a SIFT-MS instrument paired with a multipurpose autosampler (MPS Robotic Pro, GERSTEL; Mülheim, Germany). GERSTEL’s Maestro software was used to control the autosampler. Samples were incubated for 45 minutes at 80 °C in a GERSTEL agitator.
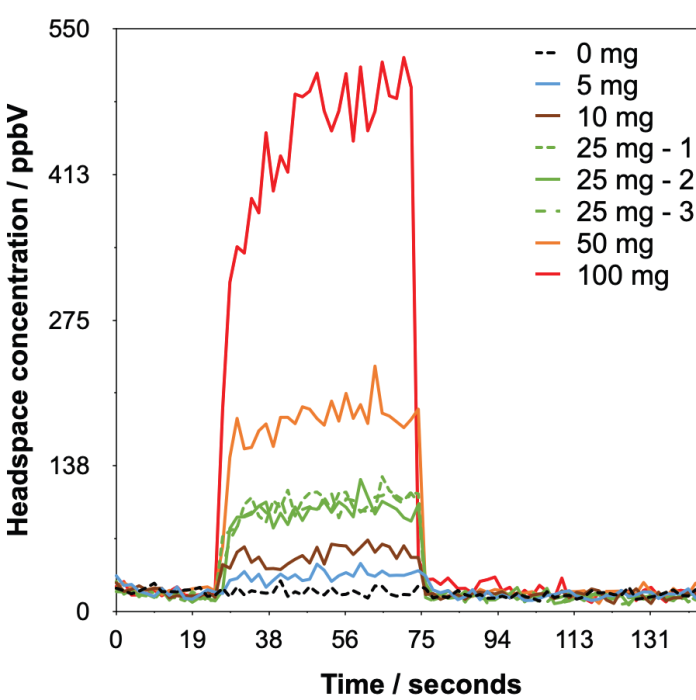
Figure 3. Example headspace injections with synchronous SIFT-MS analysis of ethylene oxide detected from Tween 80® at various dilution levels in water. Image Credit: Syft Technologies
Headspace was sampled using a 2.5 mL headspace syringe (heated to 150 °C) and thereafter injected at a flow rate of 50 μL s-1 into the SIFT-MS instrument’s autosampler inlet (warmed to 150 °C) via a GERSTEL self-sealing septumless sampling head.
Since the nominal sample flow into the SIFT-MS instrument is 420 µL s-1, a make-up gas flow (nitrogen of ultra-high purity) is also introduced via the sampling head.
The calibration procedure below automatically accounts for this dilution. The analysis time for each sample was 145 seconds (Figure 3), and the concentrations reported are the mean of the values acquired during injection (i.e., between ca. 40 and 70 seconds). It should be noted that no internal standard was applied.9
SIFT-MS Detection of Ethylene Oxide and Discrimination from Acetaldehyde
SIFT-MS freely detects ethylene oxide, as displayed in Table 1. Reaction rate coefficients, k, are the predominant measure of SIFT-MS sensitivity, and it is clear that the NO+ reagent ion is considerably less sensitive to ethylene oxide than the H3O+ and O2+ reagent ions. Thus, the NO+ product ion (m/z 74) can only be utilized at increased ethylene oxide concentrations.
Linear measurement in the gas phase is illustrated in Figure 4. Acetaldehyde is the principal potential interferent for ethylene oxide – not unlike the compendium GC-FID method,10 even though both require extremely different analytical approaches. Hence, an overview of the SIFT-MS reaction chemistry of acetaldehyde is displayed in Table 1.
Table 1. SIFT-MS reaction chemistry (rate coefficient (k), product ion formulae, branching ratio (BR as %), and mass-to-charge ratio (m/z)) for ethylene oxide (EtO) and acetaldehyde, a potential interferent. Ions in gray are not ordinarily used for quantitation because they are frequently occurring product ion m/z for other VOCs. Source: Syft Technologies
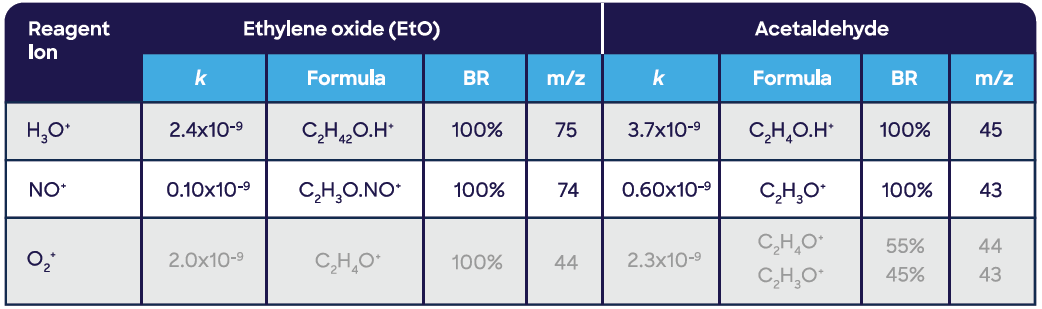
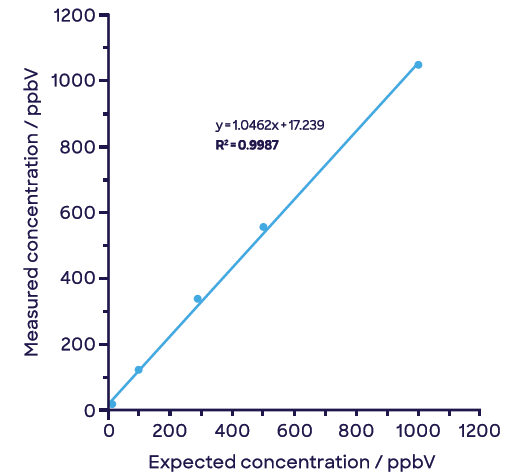
Figure 4. Linear measurement of ethylene oxide in the gas phase using SIFT-MS. Image Credit: Syft Technologies
As Tween 80® samples usually possess low levels of ethylene oxide – and particularly as this work is aimed at leveraging the maximum sensitivity to limit sample size and mitigate matrix effects – a subtraction approach is applied.
The H3O+ reagent ion is utilized to measure the combined concentration of ethylene oxide and acetaldehyde. NO+ is used to quantify the acetaldehyde concentration, allowing for the determination of ethylene oxide by subtraction of the latter value from the former.
Figure 5 illustrates how these compounds can be easily separated with this approach. The negligible signal of acetaldehyde remains so with increasing concentrations of ethylene oxide (Figure 5(a)) and vice versa with increasing concentrations of acetaldehyde (Figure 5(b)).
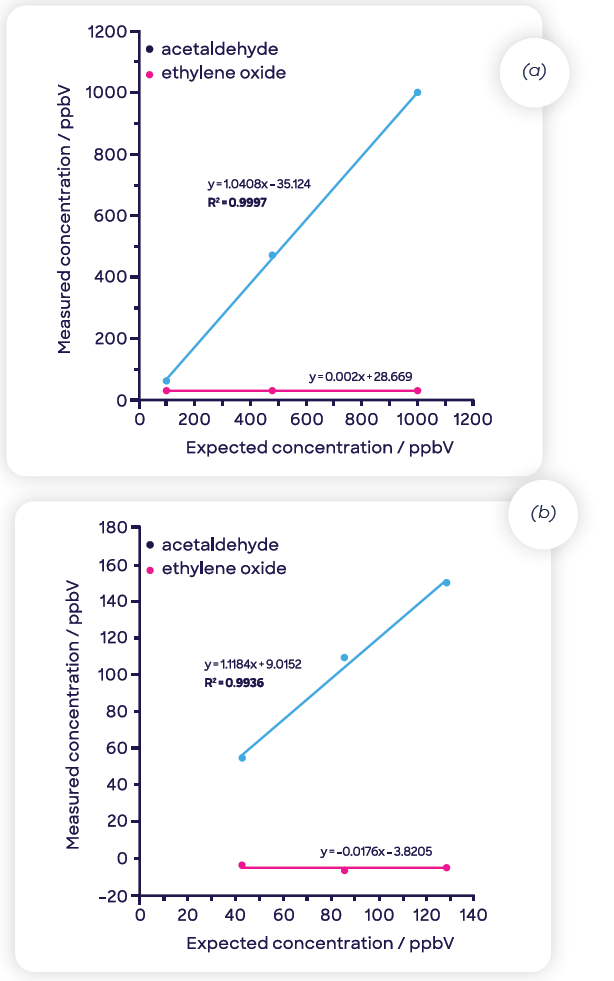
Figure 5. Effective discrimination and linear detection of (a) ethylene oxide and (b) acetaldehyde in samples containing both compounds. Image Credit: Syft Technologies
Samples
The commercial Polysorbate 80 product used in this study was Tween 80® (Croda, Inc., Edison, New Jersey).
Compared to the compendium method,11 which utilizes 1 g of sample per test, the quantity of Tween 80® applied, in this case, ranged from 5 to 100 mg, with water balance to supply a total sample volume of 1 mL.
The decision to reduce the sample volume to 1 mL was selected over the standard USP sample volume of 2.2 mL because incubation times and headspace equilibration had been optimized to accommodate the lower volume.
Results and Discussion
In order to determine the limit of quantitation for SIFT-MS analysis of ethylene oxide partitioning from Tween 80® and water mixtures, analysis of small quantities of Tween 80® in water was conducted.
The SIFT-MS results are displayed in Figure 3 and indicate clearly that ethylene oxide can be detected above baseline even when just 5 mg of Tween 80® is used (the signal-to-noise ratio is 5.0).
Analysis of triplicate samples was performed at 25 mg, offering a relative standard deviation (RSD) of 4.5%, which is in line with other headspace-SIFT-MS studies.12
Figure 6(a) displays the ethylene oxide response acquired using headspace-SIFT-MS as the mass of Tween 80® is varied (5 to 94 mg). As the matrix composition fluctuates as the mass of Tween 80® increases, the quadratic fit indicates that even at the lower dilution levels (50 and 100 mg), there may be a matrix effect.
Figure 6(b) exhibits a linear fit (regression coefficient, R2, of 0.996) acquired at higher dilutions (5 to 25 mg of Tween 80®), illustrating that in this range, the matrix effects are removed and that the system resembles the consistency of water. These high dilution levels are well-suited to SIFT-MS but not for GC-FID due to the greater sensitivity of the SIFT-MS technique.
Figure 6. Ethylene oxide concentrations for Tween 80® diluted in water determined using headspace-SIFT-MS analysis (a) across the full dilution range and (b) at the three highest dilution levels (where matrix effects are eliminated). Image Credit: Syft Technologies
Figure 7 highlights the mean of triplicate ethylene oxide measurements of the 25 mg Tween 80® sample in water and the aqueous 100 ng mL-1 ethylene oxide standard. Using the single-point calibration in water, 8.47 ng of ethylene oxide was obtained for the 25 mg Tween 80® sample, yielding a concentration in the Tween 80® sample of 339 ng g-1 (or 0.339 ppm (w/w)).
The matrix effects did not need to be adjusted as they were negligible at these comparatively high dilution levels in water.
It is presumed, therefore, that when conducting analysis of ethylene oxide in Polysorbate 80 with headspace-SIFT-MS, only a 10 mg sample needs to be introduced to 1 mL of water, with calibration performed against an external standard prepared in water.
This removes the need to purify PEG for matrix-matching the calibration standard when utilizing GC-FID, significantly reducing the sample preparation for the analysis.
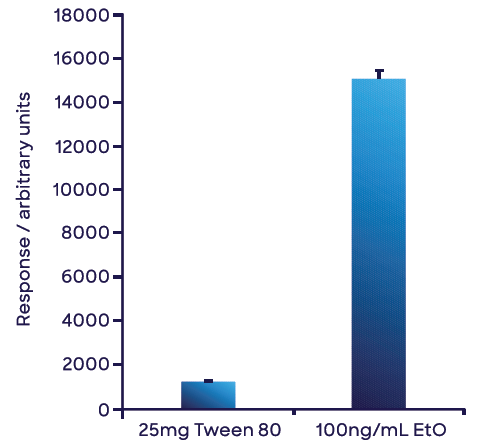
Figure 7. Measurement of the concentration of ethylene oxide in Tween 80® based on a single point external calibration. Both solutions were prepared in water. The small volume of Tween 80® used negated any matrix effects from PEG. The error bars represent 1 standard deviation of triplicate measurements. Image Credit: Syft Technologies
Conclusions
A high-sensitivity headspace-SIFT-MS analysis can detect ethylene oxide in 100-fold lower sample mass, facilitating the elimination of the 6-hour preparation that is needed for matrix-matched standards.
Headspace-SIFT-MS analysis of ethylene oxide is 9- to 14-fold faster than the compendium GC-FID method, generating sample throughputs of up to 224 samples a day.
By eliminating matrix-matched standards and delivering rapid analysis, SIFT-MS is able to supply the first test result eight-fold faster when compared to GC-FID. Simple sample preparation, ease of use, and industry-proven technology make SIFT-MS well-prepared to take on the QA/QC lab and process line.
References
- Wikipedia 1. Ethylene oxide. https://en.wikipedia.org/wiki/ Ethylene_oxide. (Accessed September 20, 2022.)
- Qiu Q-Q, Sun W-Q, Connor J (2017). Sterilization of Biomaterials of Synthetic and Biological Origin. Comprehensive Biomaterials II, 4, 180-199. DOI: 10.1016/B978-0-12-803581-8.10186-9.
- Aronson JK (2016), Ethylene oxide. In Meyler’s Side Effects of Drugs, 16th ed., Elsevier, pp 198-202. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780444537171007137.
- Wikipedia 2. Polysorbate 80. https://en.wikipedia.org
- USP (2015). Polysorbate 80. https://www.uspnf.com/notices/polysorbate-80. (Accessed September 20, 2022.)
- USP (2013). 〈228〉 Ethylene Oxide and Dioxane.
- Smith D, McEwan MJ, Španěl P (2020). Understanding gas phase ion chemistry is the key to reliable selected ion flow tube-mass spectrometry analyses. Anal. Chem. 92, 12750- 12762. DOI: 10.1021/acs.analchem.0c03050.
- Hera D, Langford VS, McEwan MJ, McKellar TI, Milligan DB (2017). Negative reagent ions for real time detection using SIFT-MS. Environments 4, 16. DOI: 10.3390/environments4010016.
- Perkins MJ, Langford VS (2021a). Application of routine analysis procedures to a direct mass spectrometry technique: selected ion flow tube mass spectrometry (SIFT-MS). Rev Sep Sci. 3, e21003. DOI: 10.17145/rss.21.003.
- Perkins MJ, Langford VS (2022). Multiple Headspace Extraction- [SIFT-MS]. Part 1: A Protocol for Method Development and Transfer to Routine Analysis. Rev Sep Sci. 4(1), e22001. DOI: 10.17145/rss.22.001.
- Perkins MJ, Langford VS (2021b). Standard validation protocol for selected ion flow tube mass spectrometry methods applied to direct headspace analysis of aqueous volatile organic compounds. Anal Chem. 93, 8386-8392. DOI: 10.1021/ acs.analchem.1c01310.
- EMA (2001). Note for guidance on limitations to the use of ethylene oxide in the manufacture of medicinal products. https://www.ema.europa.eu/en. (Accessed September 20, 2022.)

This information has been sourced, reviewed and adapted from materials provided by Syft Technologies.
For more information on this source, please visit Syft Technologies.