Sponsored by Xenocs SASReviewed by Louis CastelJan 6 2023
Batteries are omnipresent, making it hard to picture modern life without them. Throughout the world, the rechargeable batteries market value has seen a quick increase over the past few years. This has been impelled by the global awareness of environmental safety, the expanding electric vehicle market, and the assistance displayed by governments worldwide.1
Lithium-ion batteries have seen the greatest growth2 and are the most utilized rechargeable batteries due to their cost benefits and excellent technical performance (highest power density and energy, smaller and lighter compared to other rechargeable batteries, and several charges and discharge cycles in the battery’s lifespan).1
However, the lithium output might not be able to keep up with demand from battery producers, which has been expected to double over the next 10 years as global electric vehicle adoption increases. Earlier, this variance manifested as a surge in the costs of raw materials, battery-grade lithium carbonate notably, for the last two years.3
It is imperative to progress the knowledge and development of alternative battery chemistry to neutralize the global dependency in one type of technology and the reduction of vital materials.
Sodium-ion batteries (NIBs) are the most hopeful large-scale energy storage alternative due to the natural profusion and extensive distribution of raw materials, cheapness, and excellent materials sustainability relative to lithium-based technologies.4
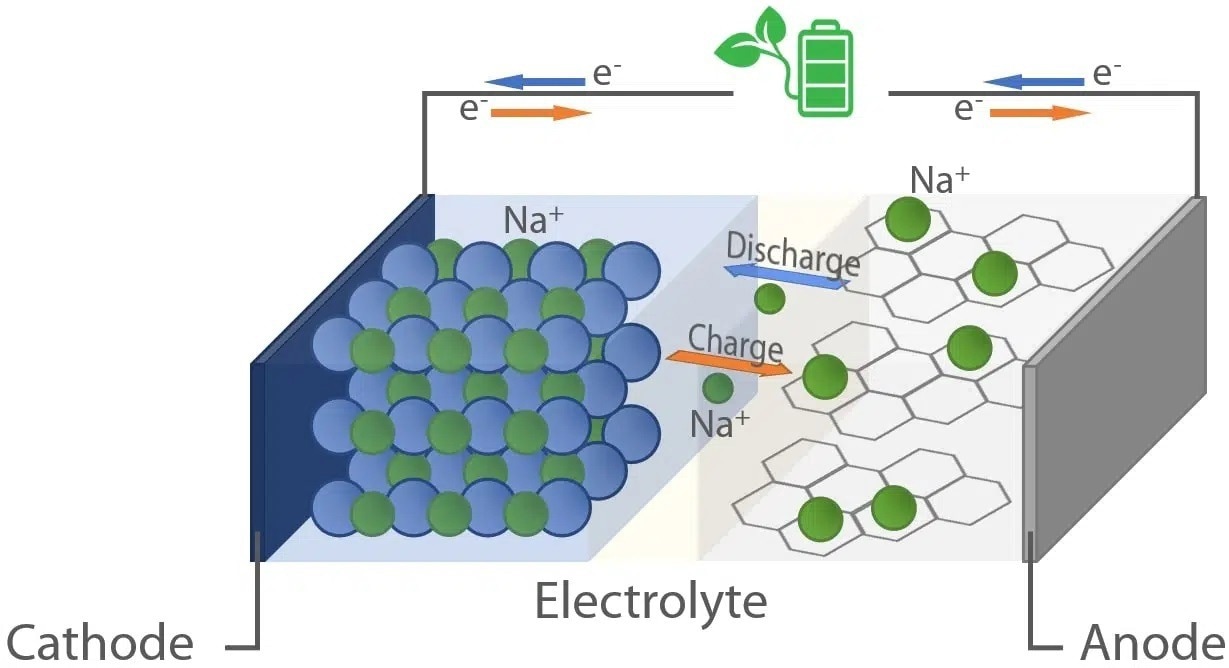
Figure 1. Schematic representation of a Na-ion battery cell. Image Credit: Xenocs SAS
The working principle and cell design of NIBs are analogous to the operating mode of lithium-ion batteries, with the fundamental difference that the charge carriers are sodium ions (Na+).
As depicted in Figure 1, a rocking chair mechanism stores energy by converting chemical and electrical energy into each other. At the time of charging, electrical energy is utilized to encourage the movement of electrons from the cathode to the anode.
Simultaneously, charge neutrality is retained by the discharge of sodium ions from the cathode and their migration to the anode via the electrolyte. The reverse process takes place during discharge.
From a design perspective, Na displays of a much larger ionic radius (1.02 Å) compared to Li (0.76 Å), making it hard to determine the correct anode materials for hosting the Na+ ions that enable a quick insertion and de-insertion.
Since the most utilized anode material in lithium-ion batteries, graphite, does not support sodium intercalation, a significant amount of research has concentrated on developing other ideal electrodes.
As a result of their low costs and high abundance, carbon-based anode materials are considered one of the most hopeful systems for NIBs.5 To achieve a high reversible capacity, most designs focus on making anode materials with a significant concentration of micropores available only to Na+ ions.
Sieving carbons (composed of highly tunable nanopores with tightened pore entrance), biomass-derived hard carbon, or hard carbons with a customized pore network are only a few instances of suggested high-energy carbon anodes.6,7,8
Therefore, it is necessary to learn about the pores network to comprehend and improve sodium ion storage. For this to be done, small angle X-Ray scattering (SAXS) is an outstanding probing method that could identify the pore-size and pore-size distribution of open and closed pores.
Such information is unavailable using other methods, like gas absorption analysis. The filling of the pores could be tracked via SAXS measurements.
Sieving Carbons
Porous carbons (PC) are inappropriate anode materials for sodium-ion batteries as their big surface area pores are also available to electrolytes which induce the formation of an unwanted solid electrolyte interphase (SEI) present within the nanopores.9
For this problem to be resolved, sieving carbons (SC), along with a tightened pore entrance (< 0.4 nm), display excellent electrochemical performance.6
SAXS measurements have disclosed that the filling of the pores with SEI could be avoided by depositing methane on a commercial PC. This helps tighten the pore entrance while retaining a similar body diameter and pore surface area.
As displayed in Figure 2, the nanopores SAXS signal of SC is changeless at the time of the cycling, while the PC signal displays the vanishing of a broad peak in the intermediate q range marking a filling of the pores with SEI.
Sieving carbons considerably surpass porous materials in terms of electrochemical performance. At 50 mA/g current density, PC show a 39 mAh/g reversible capacity, while SC display a much greater reversible capacity of 328 mAh/g.
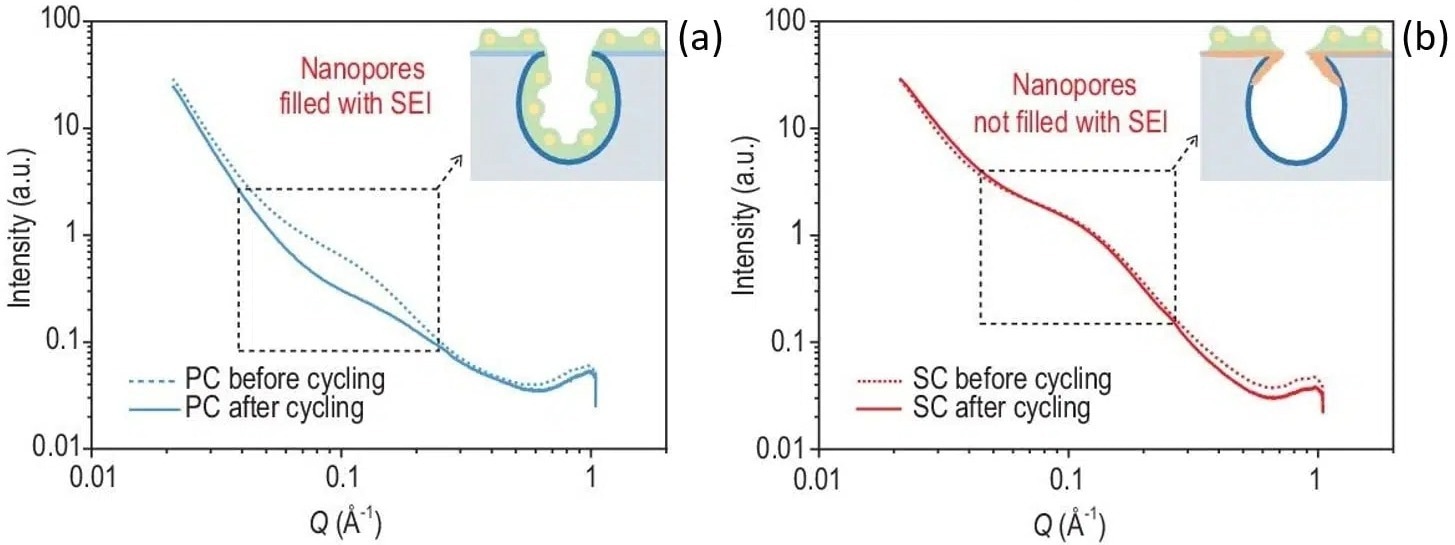
Figure 2. SAXS patterns of (a) porous carbon and (b) sieving carbon anodes recorded before and after 5 cycles at a current density of 50 mA/g. The insets display the tightened pore entrance of SC (orange) together with a representation of the solid electrolyte interphase (SEI) distribution (green) with respect to the pores. Credit: National Science Review, 2022, DOI: 10.1093/nsr/nwac084. Image Credit: Xenocs SAS
Hard Carbons with a Tailored Bimodal Pore Network
Tunning the texture of carbons is a strategy that has been suggested to improve their performance as anodes for NIBs.8 Hard carbons with a bimodal pore network of internal micropores interconnected via mesopores have been solvothermally made.
The second mesoporous network aims to improve the connectivity between micropores, easing the Na+ diffusion and eventually increasing the battery’s capacity.
SAXS measurements have shown that the size of micro and mesopores could be tuned by altering the pyrolysis temperature. As displayed in Figure 3 (a), higher temperatures generate pores of bigger diameters. This increase in size has been correlated with excellent capacity retention, as can be deduced from Figure 3 (b).
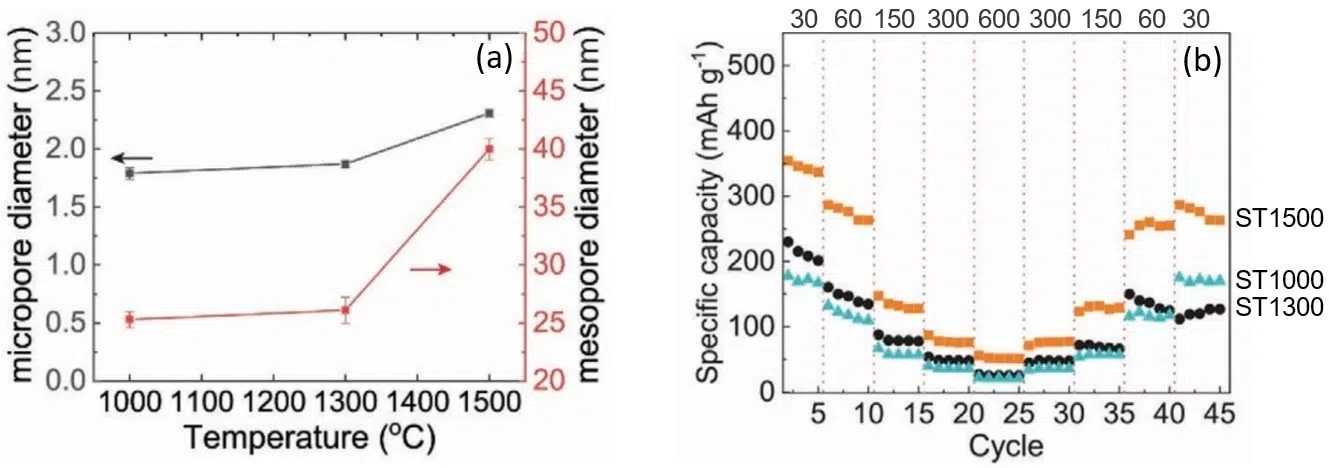
Figure 3 (a). Micro and mesopores diameters as a function of pyrolysis temperature as determined by SAXS. (b) Rate performance of discharge capacities at various current densities (indicated in the upper part of the figure in units of mA/g) for carbon anodes pyrolyzed at 1000, 1300, and 1500 oC. Credit: Advanced materials interfaces, 2022, DOI: 10.1002/admi.202101267. Image Credit: Xenocs SAS
Biomass-Derived Hard Carbon
Hard carbons derived from biomass have received significant attention as they could increase the sustainability of battery materials. In addition to being affordable, plentiful and accessible, such materials frequently tend to display ideal macro- and micro-architecture and scalable manufacturing techniques.
Rosewood, a by-product of the furniture industry, is a naturally porous material that displays great potential as anode material for sodium-ion batteries.7
With the help of simple chemical treatment, part of the lignin and the hemicellulose could be removed. Following calcination at a comparatively low temperature (1100 oC), hard carbons with a higher number of closed pores and decreased wall thickness (favoring Na+ diffusion) could be achieved.
SAXS measurements showed that chemically treated samples (ChT-1100) consist of a higher quantity of closed pores compared to the untreated parent compound (UnT-1100). As visible in Figure 4 (a), the SAXS pattern of ChT-1100 displays a highly pronounced plateau in the intermediate q region suggestive of a greater pore content.
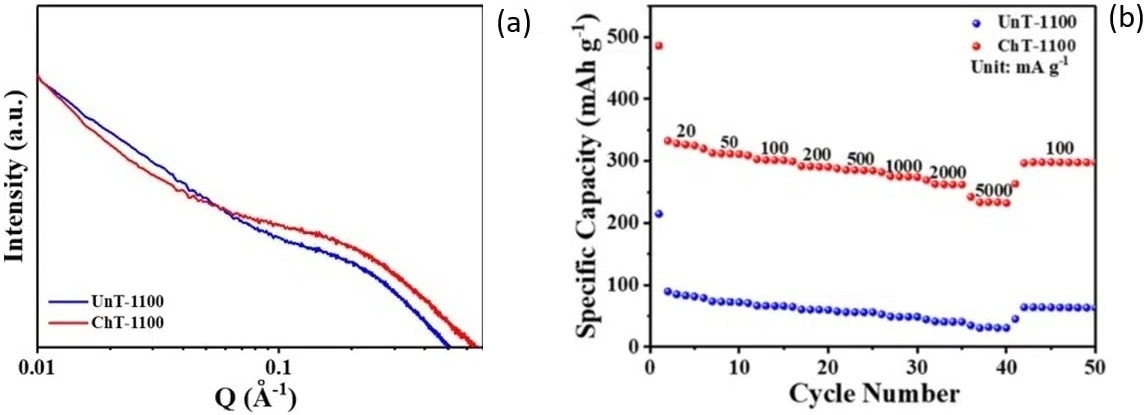
Figure 4 (a). SAXS patterns of chemically treated (ChT-1100) and untreated (UnT-1100) rosewood samples. (b). Rate performance of discharge capacities at various current densities (indicated in the figure in units of mA/g) for chemically treated and untreated rosewood samples. Credit: SusMat, 2022, DOI: 10.1002/sus2.60. Image Credit: Xenocs SAS
Regarding electrochemical performance, it is clear from Figure 4 (b) that chemically treated materials tend to exhibit a considerably enhanced reversible capacity.
Conclusions
The development of effective anode materials is a vital step in improving sodium-ion batteries’ performance. The work presented here stresses the ability of various carbon-based anode materials to offer high reversible capacities and good sodium storage.
Since the closed pores are known to be the primary microstructure housing Na+ ions, comprehending and eventually developing a suitable pore architecture is critical for producing high-energy carbon anodes.
In this context, SAXS has proven extremely beneficial in characterizing the pore structure, as well as disclosing the size and number of pores and their state (filled or empty).
References
- Rachid Amui, Janvier Nkurunziza, COMMODITIES AT A GLANCE Special issue on strategic battery raw materials. United Nations Conference on Trade and Development, 2020.
- Pillot Christophe, The Rechargeable Battery Market and Main Trends 2018-2030. AVICENNE ENERGY, 2019.
- Fitri Wulandari, Lithium price forecast: Will the price keep its bull run?, Capital, July 9, 2022, https://capital.com/lithium-price-forecast.
- Alptekin, Hande, Heather Au, Emilia Olsson, Jonathon Cottom, Anders CS Jensen, Thomas F. Headen, Qiong Cai, Alan J. Drew, Maria Crespo Ribadeneyra, and Maria‐Magdalena Titirici. “Elucidation of the Solid Electrolyte Interphase Formation Mechanism in Micro‐Mesoporous Hard‐Carbon Anodes.” Advanced materials interfaces, 9, no. 8 (2022): 2101267.
- Palaniyandy, Nithyadharseni, and Mesfin A. Kebede. “Sodium-ion battery anode materials and its future prospects and challenges.” Electrochemical Devices for Energy Storage Applications (2019): 41-58.
- Li, Qi, Xiangsi Liu, Ying Tao, Jianxing Huang, Jun Zhang, Chunpeng Yang, Yibo Zhang et al. “Sieving carbons promise practical anodes with extensible low-potential plateaus for sodium batteries.” National Science Review (2022).
- Zhou, Siyu, Zheng Tang, Zhiyi Pan, Yuancheng Huang, Le Zhao, Xi Zhang, Dan Sun, Yougen Tang, Abdelghaffar S. Dhmees, and Haiyan Wang. “Regulating closed pore structure enables significantly improved sodium storage for hard carbon pyrolyzing at relatively low temperature.” SusMat (2022).
- Alptekin, Hande, Heather Au, Emilia Olsson, Jonathon Cottom, Anders CS Jensen, Thomas F. Headen, Qiong Cai, Alan J. Drew, Maria Crespo Ribadeneyra, and Maria‐Magdalena Titirici. “Elucidation of the Solid Electrolyte Interphase Formation Mechanism in Micro‐Mesoporous Hard‐Carbon Anodes.” Advanced materials interfaces, 9, no. 8 (2022): 2101267.
- Li, Kaikai, Jun Zhang, Dongmei Lin, Da-Wei Wang, Baohua Li, Wei Lv, Sheng Sun et al. “Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes.” Nature communications, 10, no. 1 (2019): 1-10.

This information has been sourced, reviewed and adapted from materials provided by Xenocs.
For more information on this source, please visit Xenocs.