Droplet size distribution (DSD) is a key parameter of emulsions. Determining DSD is essential for quality control, product evaluation at various stages of processing, manufacturing, and production, as well as for assessing the properties of novel formulations in research and development.
Numerous industries require straightforward and precise determination of DSD in various emulsions without disrupting the sample structure. An emulsion is a mixture of two or more immiscible liquids that form a system of dispersed droplets (dispersed phase) separated by the continuous phase matrix.
Examples include margarines, butters, food dressings, paints, and crude oil. Emulsions are generally stable and maintain their structure (i.e. the droplets do not coalesce) for extended periods and under specific conditions (e.g. temperature).
This article demonstrates how MQC-R, a research-focused time domain, nuclear magnetic resonance (NMR) spectrometer, can be employed for routine determination of DSD in emulsions, in this case in a margarine sample, and how it can be applied in a quality control environment.
The Time Domain NMR Approach
The Time Domain NMR approach for DSD analysis is based on the phenomenon of restricted diffusion. The effective mobility of the dispersed phase molecules moving within the droplets is substantially lower than that of equivalent bulk liquids due to interactions with droplet walls. Consequently, droplet size information can be obtained through NMR diffusometry measurements.
Raw data is collected in the form of NMR signal diffusion decays. In an emulsion sample containing both continuous and dispersed phases, each phase generates an NMR signal.
To obtain NMR data only for the dispersed phase, the continuous phase signal is removed using a suitable T1 relaxation filter, followed by a pulsed-field gradient (PFG) diffusometry protocol. Signal selection is further enhanced by maintaining a constant sample temperature using a variable temperature (VT) NMR sample probe. Under these conditions, the effective T1 relaxation time of the continuous phase is much shorter than that of the dispersed phase, thereby improving the efficiency of the T1 filter.

Image Credit: Robyn Mackenzie /Shutterstock.com
Advantages of using MQC-R for analysis of droplet size distribution:
- Simple and dependable method
- Straightforward sample preparation
- Non-destructive: allows repeated measurement of the same sample
- Provides droplet dimension data, instead of droplet clusters
- No hazardous solvents or waste involved
- Direct measurement of bulk samples
- Applicable across a broad range of emulsions
- Consistent results achieved by fixing the sample temperature.
Sample Preparation
For each emulsion formulation, three samples must be prepared:
- A pure sample of the continuous phase
- A pure sample of the dispersed phase
- An emulsion sample or set of emulsion samples composed of both phases. Additionally, a deionized water sample may be required for calibration.
In this example, using margarine, the following three samples were prepared: (1) dispersed phase inside the droplets (water-based), (2) continuous phase (fat-based), and (3) emulsion - margarine. After preparation, the samples were transferred into NMR tubes and conditioned at +5 °C, the typical temperature used when determining DSD of dairy products.
Calibration Procedure
Calibration involves three automated steps:
- Adjustment of T1 filter parameters to remove the continuous-phase NMR signal
- Collection of diffusion data for pure (deionized) water to adjust effective PFG strength
- Collection of diffusion data for the pure dispersed phase to establish initial parameters for data processing.
Analysis Procedure
Following instrument calibration for samples containing the same continuous and dispersed phases, diffusion data can be routinely collected, without additional refinement, from emulsion samples equilibrated at the same temperature used during calibration. This data is then used to determine DSD results.
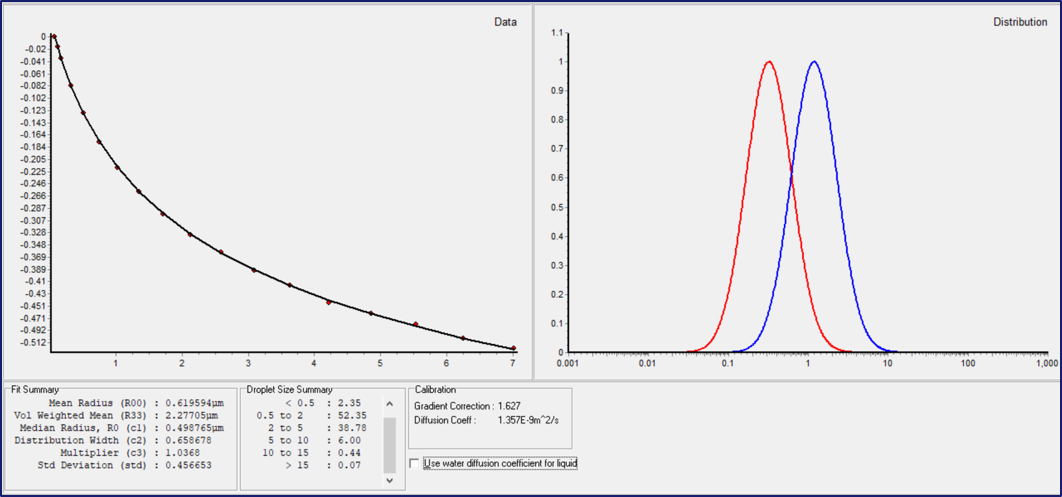
Figure 1. The DSD results obtained using the MQC-R after the fitting of NMR diffusometry data for a margarine sample. The experimental diffusion decay (•, the red solid circles) and the log-normal fitting curve ( – , the solid black line) are shown in the left-hand graph window. The volume-weighed droplet size distribution ( – , the blue curve) and the mean size distribution ( – , the red curve) are plotted in the right-hand graph. The numerical parameters of the distribution are listed in the “Fit Summary” and “Droplet Size Summary” tables below. Image Credit: Oxford Instruments
Results
Data processing is performed using a log-normal distribution of diffusion coefficients.
The fitting results are displayed both as a distribution function graph and as a set of the distribution function parameters (Figure 1): the mean droplet radius (R00), the volume-mean radius (R33), the median radius (R0), the distribution width (σ), and the percentage of droplets within a user-specified, predefined radius range.
Tables 1 and 2 present the repeatability of the DSD measurements obtained with the MQC-R.

Image Credit: Oleksiy Mark /Shutterstock.com
Table 1. Repeatability of DSD measurements obtained for the margarine sample using the MQC-R. Source: Oxford Instruments
|
Droplet size distribution parameters
|
Repeat 1
|
Repeat 2
|
Repeat 3
|
Average value for three repeats (μm)
|
Standard deviation for three repeat measurements (μm)
|
| Mean radius, (μm) |
0.61 |
0.54 |
0.52 |
0.56 |
0.04 |
| Volume weighed mean, (μm) |
2.31 |
2.38 |
2.35 |
2.35 |
0.03 |
| Median radius, (μm) |
0.49 |
0.43 |
0.40 |
0.44 |
0.04 |
| Distribution width, (μm) |
0.67 |
0.70 |
0.71 |
0.69 |
0.02 |
Table 2. Repeatability of measurement of the content of droplets with a specific radius using the MQC-R for margarine. Source: Oxford Instruments
|
Droplet radius ranges*
|
Percentage of droplets with radius within the respective range (%)
|
Average value for three repeats (μm)
|
Standard deviation for three repeat measurements (μm)
|
|
Repeat 1
|
Repeat 2
|
Repeat 3
|
| Smaller than 2 μm |
54.06 |
53.47 |
54.52 |
54.02 |
0.43 |
| From 2 to 5 μm |
39.04 |
38.40 |
37.51 |
38.32 |
0.63 |
| From 5 to 10 μm |
6.33 |
7.28 |
7.11 |
6.91 |
0.41 |
| From 10 to 15 μm |
0.49 |
0.70 |
0.70 |
0.63 |
0.10 |
| From 15 to 30 μm |
0.09 |
0.15 |
0.15 |
0.13 |
0.03 |
| Larger than 30 μm |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
*The number of the radius ranges and their numerical borders can be defined by the user.
Conclusion
The MQC-R offers a non-destructive and dependable technique for determining droplet size distribution in emulsions, enabling straightforward monitoring of product properties and consistency. Sample preparation is simple and does not require hazardous solvents or contrast additives.
Furthermore, maintaining constant sample temperature during analysis preserves emulsion structure. In addition, the NMR method is non-destructive, allowing for repeated analysis of the same sample before being used elsewhere.
Ultimately, MQC-R enables more definitive and precise formulation of novel emulsions, along with improved manufacturing consistency and enhanced process control, resulting in reduced expenses.

Image Credit: Oxford Instruments

This information has been sourced, reviewed, and adapted from materials provided by Oxford Instruments.
For more information on this source, please visit Oxford Instruments.