The TA Instruments RS-DSC (Rapid Screening-Differential Scanning Calorimeter) is an advanced and adaptable device that transforms the thermal stability assessment of biological drugs through its exceptional efficiency and optimized analysis.
For researchers engaged in the rigorous field of biologic drug development, comprehending the stability of biomolecules under varying thermal conditions is essential for maintaining product quality and facilitating regulatory approval.
Short-term thermal stability assessments demonstrate a compound's ability to withstand thermal stress, forecast its shelf life, and confirm its efficacy. Accurately measuring this in a high-throughput setting poses significant challenges, particularly in avoiding disruptions to processes or adhering to strict timelines.
In response to this need, TA Instruments has developed the RS-DSC (Rapid Screening-Differential Scanning Calorimeter), an innovative solution designed for the swift characterization of the thermal stability of biotherapeutics.
The TA Instruments RS-DSC Can Help
Accelerate Thermal Stability Testing
The TA Instruments RS-DSC offers comprehensive insights into the thermal stability of drug products at a significantly faster rate by enabling the simultaneous analysis of up to 24 samples. The technology of the TA Instruments RS-DSC facilitates the characterization of highly concentrated drug products.
Improve Efficiency
The TA Instruments RS-DSC optimizes material usage by employing disposable MFCs (Micro Fluidic Chips) to hold the sample. MFCs require a sample volume of less than 15 µl, and the design promotes cleaner, more efficient operation by minimizing laborious sample dilution, frequent instrument cleaning, and contamination risks.
Make More Informed Decisions
NanoAnalyze™ Software seamlessly manages the high-volume data generated by the TA Instruments RS-DSC, delivering comprehensive, accurate insights into a molecule's thermal stability and thermodynamic properties.
Groundbreaking Capabilities

Image Credit: TA Instruments, Inc.
Enhanced Throughput
The TA Instruments RS-DSC enables the simultaneous analysis of 24 samples, greatly enhancing research efficiency and expediting the entry of biologic drugs into the market.
Resource Efficiency
The minimal sample volume requirements of the TA Instruments RS-DSC ensure optimal material utilization while minimizing costs.
High Concentration Proficiency
The TA Instruments RS-DSC is proficient in testing a wide range of sample concentrations and has a distinctive ability to analyze very high-concentration drug products both efficiently and effectively.
Simplified Workflow
The TA Instruments RS-DSC simplifies processes by eliminating the necessity for sample dilution when handling high-concentration samples, and the use of disposable microfluidic chips reduces or negates the need for cleaning, thereby lowering the risk of contamination.
Comprehensive Data Analysis
The NanoAnalyze Software oversees data management and delivers in-depth insights to enhance the development.
Features and Benefits
Parallel Analysis: The high-throughput analysis enables up to 24 concurrent measurements, thereby expediting research.
Single-Use Microfluidic Technology: MFCs enhance operational efficiency by simplifying the characterization of high-concentration drug products while minimizing cleaning time and contamination risks.
State-of-the-Art Data Analysis Software: The powerful and intuitive NanoAnalyze Software automatically and reliably analyzes data for thorough and swift evaluation.
Source: TA Instruments, Inc.
| RS-DSC |
|
| Cell Geometry |
Disposable microfluidic |
| Cell Material |
Glass |
| Sample Format |
MFC (Micro Fluidic Chips) |
| Working cell volume |
11 µL |
| Sample Capacity |
24 MFCs |
| Typical Sample Concentration |
20 mg/mL – 330+ mg/mL IgG (protein dependent)1 |
| Sample throughput |
> 96 samples/day |
| Temperature Range |
20-100 °C |
| Temperature Scan Rate |
1 or 2 °C/min |
| Temperature accuracy |
± 0.2 °C (across all calorimeters); ± 0.1 °C reproducibility2 |
1 Using lysozyme in 0.1 M glycine buffer at pH 2.5 at 1 °C/min
2 Using DPPC in water at pH 7 at 1 °C/min Accelerate research, improve efficiency, and make more informed decisions with the TA Instruments RS-DSC, a revolutionary new high-throughput thermal stability testing instrument specifically designed for biologic drug development.
Technology
Groundbreaking Capabilities
The TA Instruments RS-DSC transforms the field by enabling the simultaneous analysis of 24 samples, a significant advancement over traditional single-sample methods. It operates with less than 15 µL per sample, representing a considerable decrease compared to standard capillary DSCs, and typically provides a clearer understanding of the thermodynamics of unfolding than differential scanning fluorescence (DSF).
Microfluidic Technology: The Future of Precision and Convenience
Fitted with state-of-the-art Micro Fluidic Chips (MFCs), the TA Instruments RS-DSC is engineered to hold the sample with ease. This technological advancement eliminates the need to repeatedly clean the instrument's measurement cell between analyses, conserving time, minimizing contamination risks, and enabling more accurate and reliable readings.
The MFCs are designed for single-use and improve operational efficiency, enabling swift transitions while protecting the instrument from harmful substances. The innovative MFC design represents advanced, low-volume, single-use technology that simplifies sample loading and preparation using standard laboratory tools. A sample can be prepared, sealed, and set for analysis in under one minute, requiring only minimal volumes for accurate assessment.

Image Credit: TA Instruments, Inc.
Application
The TA Instruments RS-DSC is ideal for a wide range of applications in biologic drug development, including:
Formulation Buffer Screening
Thermal stability is a crucial indicator of a biologic drug product's overall clinical efficacy, and Differential Scanning Calorimetry (DSC) is a key instrument for assessing the influence of the solution environment on protein stability. Variations in protein stability may manifest as minor shifts in Tmax or as significant changes of several degrees, due to modifications in factors such as pH, buffer composition, ionic strength, excipients, and detergents.
To illustrate how formulation screening data can assist in the selection of buffer components, the antibody trastuzumab was evaluated under four typical buffer conditions:
- A standard working buffer (PBS)
- A buffer that facilitates lysine conjugation for the synthesis of labeled antibodies for cellular trafficking studies or drug conjugation (borate)
- A trastuzumab-based antibody-drug conjugate (succinate)
- The native formulation buffer for trastuzumab (histidine)
The initial unfolding event, corresponding to the unfolding of the CH2 domain, is not significantly affected by histidine, borate, or PBS buffers. In contrast, the succinate buffer causes destabilization of the CH2 domain, leading to a decrease in the onset of unfolding and Tmax,1 by approximately 3 °C.
Regarding the primary transition that reflects the unfolding events of the Fab and CH3 domains, the histidine and succinate buffers exhibit the highest stabilizing effects, with a Tmax, 2 of 82.66 °C. The main transition is least stable in the borate buffer, which has a Tmax, 2 of 80.69 °C. As expected, the most stabilizing buffer formulation for trastuzumab in this sample set is the histidine buffer utilized for the final formulation of the approved drug product.
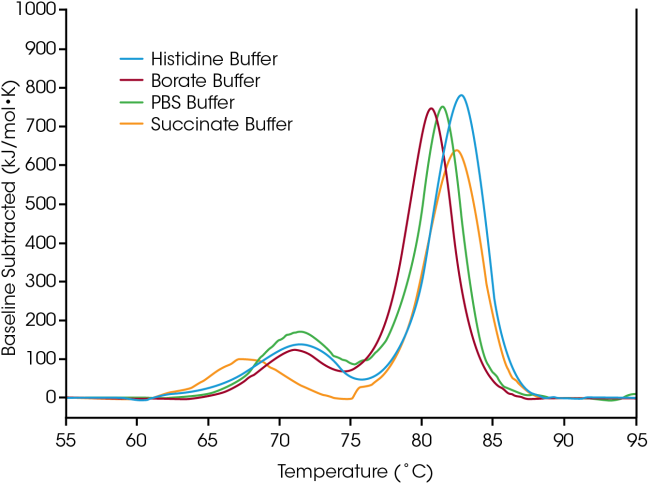
Image Credit: TA Instruments, Inc.
Protein Mutational Analysis – Characterize Engineered Protein Modifications to Understand the Structural Impact on Molecular Stability
Protein mutations are a prevalent approach for enhancing protein structure and functionality, with even minor amino acid alterations potentially leading to significant changes in overall protein stability. Employing Differential Scanning Calorimetry (DSC) to characterize engineered protein modifications is crucial for understanding the structural implications of mutations on the protein as a whole and for informing decision-making in the biologic drug development pipeline. A small panel of engineered proteins was evaluated for variations in thermal stability resulting from single amino acid mutations within the protein sequence to illustrate the effects that sequence modifications can exert on stability.
In the parent protein, unfolding occurs over a single major thermal transition at Tmax = 75.92 °C. A single amino acid mutation (Mutation 1) that does not significantly affect short-term thermal stability was introduced; however, alternative single amino acid mutations (Mutations 2 and 3) have been shown to exert considerable effects on protein stability.
As evidenced by the notable destabilization observed in Mutation 3, modifications do not uniformly yield the same outcomes; rather, their effects depend on both the location of the modification and the physicochemical properties of the substituted amino acid. Striking a balance between the desired functional advantages of sequence modifications and the structural stability of the protein as a whole enhances the understanding of the structure-function relationship and can promote the development of advanced therapeutics.
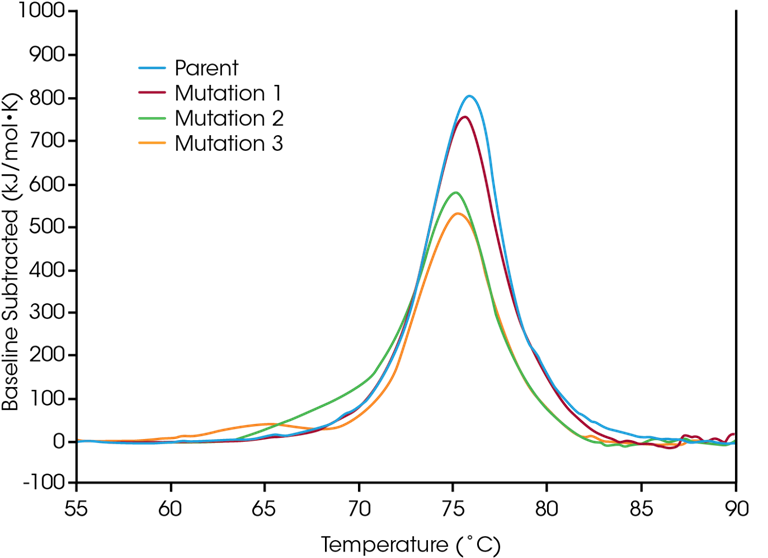
Image Credit: TA Instruments, Inc.
Concentration Dependence – Investigate Stability Changes in Highly Concentrated Drug Products
The TA Instruments RS-DSC is specifically engineered to accommodate high-concentration biologic drug samples, particularly focusing on antibody drugs and antibody drug conjugates. Given the increasing success of antibody therapeutics, there is a heightened interest within the pharmaceutical industry for high-concentration dosage forms that facilitate subcutaneous and ocular drug delivery.
Formulations with antibody concentrations ranging from 50 to 150 mg/mL are prevalent, with some exceeding 200 mg/mL. However, formulating proteins at elevated concentrations may heighten their vulnerability to physical instability. On the other hand, some case studies have shown improved thermal stability at higher concentrations. Therefore, comprehending thermal unfolding and the response to the solution environment at the relevant formulation concentration is an essential metric for reducing drug product liability.
To showcase the capability of testing high-concentration protein samples and to emphasize the significance of testing at the targeted formulation concentration, researchers assessed chicken egg white lysozyme within the range of 30 to 330 mg/mL in glycine buffer. At low concentrations (~1 mg/mL), lysozyme typically presents a straightforward single transition thermogram and is frequently utilized as a reference test sample for DSC. By evaluating protein concentrations up to 100 times greater, we noted a concentration-dependent effect on lysozyme stability.
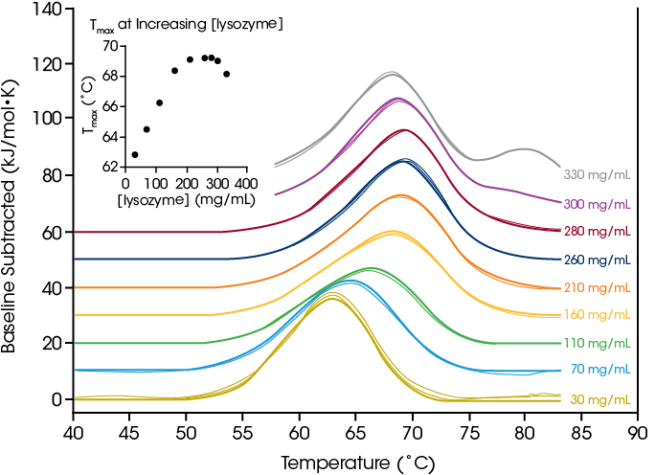
Image Credit: TA Instruments, Inc.