Jan 18 2006
Topics Covered
Background
Case Studies
Analysis of a Multi-Layer Polymer Coating
Pharmaceutical Packaging Problems
Pharmaceutical White Paper-Surface Analysis Exposes Counterfeit Drugs
Background
Modern methods of surface chemical characterization play an important role in the study and development of pharmaceutical products
Lucideon offers a comprehensive range of surface analysis techniques. Some techniques available at Lucideon include:
Case Studies
Analysis of a Multi-Layer Polymer Coating
Time of Flight Secondary Ion Mass Spectroscopy (ToFSIMS) has become an important analytical tool for R &D of controlled-release drug delivery systems.
A multi-layer polymer coating, which encapsulated a drug bead, was analysed by ToFSIMS to determine the integrity of the coating and the distribution of the active drugs. Cross-sectioning the beads allowed the coating thickness to be determined.
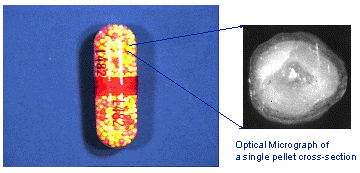
Figure 1. Optical micrograph of a controlled drug delivery bead.
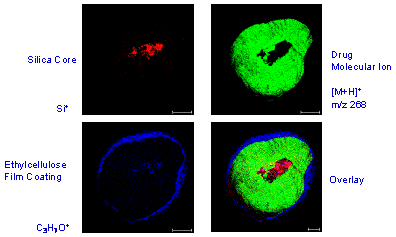
Figure 2. RGB overlay of Time of Flight Secondary ion Mass Spectroscopy images.
The coating was characterised enabling the pharmaceutical company to determine the direction of their drug development.
Pharmaceutical Packaging Problems
Product/package interactions are amongst the most critical in pharmaceutical production.
XPS (X-Ray Photoelectron Spectroscopy) was utilised to determine the surface composition and variation of composition across the surface of a glass bottle used for drug storage.
Table 1. Variations in the composition of a glass container used for drug storage (in atomic %), determined by x-ray photoelectron spectroscopy (XPS).
| |
Neck
|
Base
|
Side Wall
|
|
Silicon
|
21.8
|
23.2
|
24.9
|
|
Oxygen
|
49.4
|
52.7
|
55.4
|
|
Carbon
|
19.3
|
13.3
|
8.6
|
|
Sodium
|
3.6
|
3.9
|
4.5
|
|
Aluminium
|
2.1
|
2.1
|
2.2
|
|
Boron
|
2.8
|
3.5
|
3.8
|
|
Nitrogen
|
0.3
|
0.3
|
Not detected
|
|
Calcium
|
0.3
|
0.2
|
0.2
|
|
Potassium
|
0.6
|
0.8
|
0.5
|
The results showed the variations of the composition of the glass bottle which allowed the client to evaluate the suitability of the container.
Pharmaceutical White Paper-Surface Analysis Exposes Counterfeit Drugs
As counterfeit drugs are becoming increasingly available (the US-based Centre for Medicines in the Public Interest predicts that counterfeit drug sales will be worth an estimated US$75 billion globally in 2010), the technology that is used to manufacture these fake medicines is becoming more and more sophisticated.
Lucideon Surface and Materials Analysis, together with GlaxoSmithKline, has developed a method of identifying counterfeit tablets, when compared to genuine drugs, by utilising techniques such as XPS and ToFSIMS.
Further to normal methods of looking at composition, this method concentrates on process and the manufacturing route of the drugs, the end result being that previously undetectable chemical copies of pharmaceuticals can be identified

This information has been sourced, reviewed and adapted from materials provided by Lucideon.
For more information on this source, please visit Lucideon.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.