Nov 7 2009
Kaca Skokanova / Shutterstock
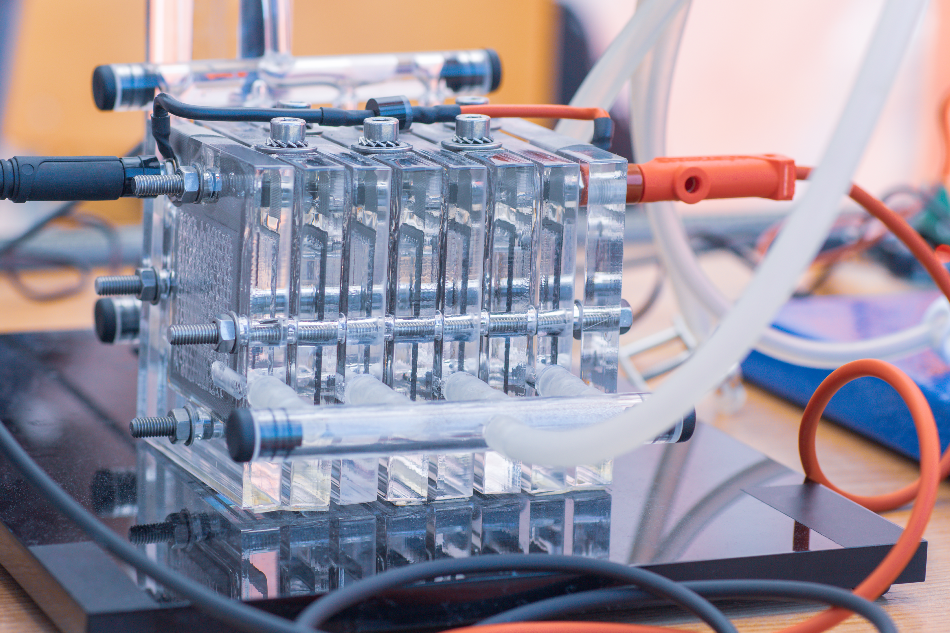
Fuel cells are currently accepted as a dependable source of power for military and space applications. They are being optimized for a wide range of applications, including vehicles. Although broadly established and technically proven, their cost requirements have to be reduced to make them really competitive in large-scale markets.
Carbon-supported gold alloy catalysts are being analyzed as alternatives for the costly platinum used within the fuel cell stack. Other gold catalysts also play a role in synthesizing pure hydrogen from fuels such as propane, natural gas, or biogas.
Fuel cells continuously transform chemical energy into electrical energy. Most of the fuel cells combine hydrogen with atmospheric oxygen, an operation that is contrary to the electrolysis of water. This electrochemical process eliminates the drawbacks of the Carnot heat cycle, and fuel cell systems can utilize a broad range of fuels a lot more efficiently than traditional generators, in addition to being silent and free of pollution.
A number of fuel cells include a range of bipolar plates usually made of a conductive material such as stainless steel. In order to realize good corrosion resistance and minimize the contact resistance between the plate and diffusion media in the cell, a thin layer of conductive gold can be utilized.
Gold in Fuel Cell Catalysis
Gold is a good oxygen reduction catalyst in alkaline electrolytes (used in Space Shuttle Orbiter fuel cells), and can also catalyze the direct oxidation of sodium borohydride with high utilization. Recently, due to a reputation for low activity in acid electrolytes, gold has virtually played no role in the development of acidic, proton-exchange membrane (PEM) fuel cells.
The need to bring down the cost of the platinum necessary to catalyze low-temperature fuel cells has resulted in the study of carbon-supported PdAu alloy anode catalysts at Lawrence Berkeley National Laboratory. Usually, carbon monoxide acts as a strong poison for platinum catalysts, but these alloys have exhibited high CO tolerance in acid electrolytes.
Under conditions of cyclic voltammetry, carbon-supported Pd80Au20 alloys seem to be about three to five times more active for CO/H2 oxidation compared to typical Pt50Ru50 carbon-supported catalysts in the sulfuric acid electrolyte (0.5 M H2SO4 in 250 ppm CO/H2 mixture). It has been proposed that palladium is stabilized by gold so that CO is not adsorbed strongly.
Electrocatalysts with reduced platinum loading and improved activity have been synthesized at Brookhaven National Laboratory. Carbon-supported Pt/Au/Ni catalysts including a core-shell of AuNi alloy nanoparticles (that is, one to two monolayers of Au around a Ni core) displayed a Pt mass-specific activity of around 20 times that of platinum/carbon catalyst in acidic electrolyte. Surface segregation of Au was confirmed using X-ray powder diffraction method.
Adzic et al. have demonstrated that carbon-supported Pt/Au catalysts are significantly more stable than Pt/C supported catalysts. Research by Zhong et al. has revealed that the Au/Pt system shows considerably different electrocatalytic properties than either Pt or Au alone. The reasons for this have been illustrated by Bond.
Research efforts are continuing to improve these catalysts for alkaline and acid fuel cells, while new methods for preparing high surface area gold alloy catalysts should offer a stimulating competitor for platinum catalysts for both acidic and alkaline electrolytes.
Top Japanese manufacturer Hitachi Maxell stated it has developed a new gold-based catalyst that can be used in oxygen reduction reaction at the cathode of polymer electrolyte fuel cells (PEFC).
The new catalyst is composed of gold-platinum (AuPt) nanoparticles, 2–3 nm in size. The new AuPt catalyst produces about 4.8 times higher oxygen reduction current per unit area compared to a commercial platinum catalyst. This success signifies a big step toward the use of fuel cells for applications necessitating large currents, such as power sources for homes and automobiles.
Gold in Hydrogen Generation and Purification
Costly catalysts are used to produce pure hydrogen for fuel cell systems, and gold is likely to have a considerable effect in decreasing costs. Huge efforts are ongoing to create compact reactors for stationary fuel cell systems and vehicles.
A majority of these include a hydrocarbon fuel reforming stage, followed by a water gas shift reaction to produce hydrogen-rich gas mixtures. These mostly comprise a small proportion of carbon monoxide, and efforts must be taken to eliminate this since it acts as a poison to fuel cell catalysts.
In the water gas shift (WGS) reactor, carbon monoxide is made to react with steam to synthesize more hydrogen:
CO + H2O → CO2 + H2
The water-gas shift reactor catalysts must realize high conversion at relatively low temperatures. A reduction in the reaction temperature leads to a decrease in the proportion of CO present in the equilibrium. Supported gold catalysts have exhibited activity comparable to CuO/ZnO/Al2O3 materials currently available in the market, with activity reported at temperatures as low as 120 °C.
The gold-based catalysts do not need any special activation steps and are resistant to start-up and shut-down cycles. Specifically, Au/CeO2-ZrO2 catalysts have shown high durability and activity.
Low-temperature polymer electrolyte membrane fuel cells can only work on reformate mixtures after the elimination of CO from the fuel gas stream. A preferential oxidation (PROX) reactor is used to inject oxygen or air (equivalent to 0.5%–1% oxygen) into the fuel stream upstream of the reactor to preferentially oxidize CO to CO2, even when hydrogen is present.
2CO + O2 → 2CO2
Gold supported on metal oxides like TiO2, Fe2O3, and Co3O4 will catalyze CO oxidation at temperatures as low as −70 °C in the presence of hydrogen. When compared to commercially available Pt/ƒÁ-Al2O3 PROX catalysts, Au/a-Fe2O3 catalysts exhibit higher activity at lower temperatures. Luengnaruemitchai et al. found that Au/CeO2 catalysts were highly effective.
In general, fuel cells have reached an impressive stage of development and are quickly expanding into various markets, and gold catalysts are making a substantial contribution to their success by offering technically better materials at lower costs.