| Take a look at a portrait of George Washington shortly after he became the first President of the United States, and the chances are he’ll look bloated around the mouth. As it turns out, Washington was often struggling to win a battle in his mouth, forcing his jaws shut against the severe steel spring that held his bulky walrus ivory dentures in place. These days things are better for denture wearers. Yet even 200 years later, everything is not as rosy as it could be. Denture Stomatitis According to recent surveys, around 40% of the adult population wear dentures. Of these, it is estimated that up to two thirds will suffer from so-called ‘denture stomatitis’ at some stage. This condition is essentially a recurring inflammation of the soft tissues that support the denture, figure 1. As to what causes it, a glance at the literature reveals that (in clinician speak) ‘the condition exhibits a complex multifactorial aetiology’. In other words, no-one’s exactly sure, but there seem to be a few factors that play a part. Among the most commonly-cited contributing factors are trauma (which may be caused by a poorly-fitting denture), poor oral hygiene and fungal infection. To top it all, there are three different kinds of denture stomatitis, that have were identified in 1962. | 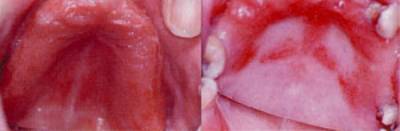
| | Figure 1. Denture stomatitis of the gums (left) and on the palate of a partial denture wearer. | Treatment of Denture Stomatitis Dentists differ in their preferred treatments for denture stomatitis. Some recommend the denture is worn less regularly or left out altogether for a time, but this is an inconvenience and understandably unpopular with patients. An alternative approach is to apply a soft polymer gel to the fitting surfaces of the denture. This temporary polymeric cushion relieves the trauma caused by the denture rubbing on the inflamed tissues, and, as it is able to flow, it will adapt as the underlying tissues heal, figure 2. The gel - called a tissue conditioner - can be discarded once the inflammation has subsided. | 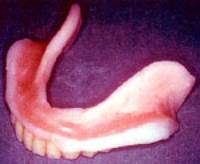
| | Figure 2. A denture with tissue conditioner fitted. | Tissue Conditioners Developing suitable gels for use as oral tissue conditioners is an interesting problem in itself. The material must of course be biocompatible. And since polymers in any moist environment are prone to leach their constituents, these should he non‑toxic, unleachable, or both. On the flip side of the coin, polymers often absorb moisture easily. In the mouth, this can lead to food debris and oral microbes getting into the material and fouling it, so low absorption is important. The gel must also have suitable viscoelastic properties so that it will deform in preference to the underlying tissues -i.e. perform its cushioning role - yet flow to adopt the changing contours of the traumatised tissue as it heals. A fourth requirement ensures that neither the dentist is overly-taxed, nor the patient overly-bored - it must be simple to apply and form a working tissue conditioner in situ in a matter of minutes. Poly(ethyl methacrylate) Gels Traditionally, tissue conditioners have been made by mixing a low methacrylate powder, such as poly(ethyl methacrylate), PEM, with a liquid consisting of a large ester plasticiser and 8-50% ethanol. The ethanol disrupts the Van der Waals’ bonds in the polymer particles, making then swell, thereby encouraging the ingress of plasticiser. Gels made in this way gain their mechanical strength by entanglement of the polymer chains, so there are no polymerisation exotherms or dubious by-products to worry about. The dentist simply mixes the powder and liquid at the chairside applies the gel to the denture and returns the denture to the patient. That may sound all well and good on paper, but in practice, the ethanol leaches out of the gel within a few hours. As well as the toxicity issue this raises, myth holds that at least one very sober patient has suffered the ignominy of failing a breathalyser test shortly after leaving their dentist's surgery. The most common plasticisers used are phthalates. These also leach into the mouth, and a forest of literature has been written on the toxicity of these chemicals. It is plain then that in terms of biocompatibility, there is plenty of room for improvement. Oral Infections A tissue conditioner is often only part of the treatment used to alleviate denture stomatitis. Accompanying many cases is an oral infection, where the offending microbe is the commensal oral yeast, Candida albicans. To clear up the infection, a patient may be prescribed antifungal lozenges or an antifungal mouthwash, that must be used four times a day with the dentures removed. Being inconvenient, this type of regime often falls foul of poor patient compliance, and administering the drug in this fashion leads to inefficient, cyclic levels of drug in the mouth. A possible solution would be to combine the drug into the tissue conditioner so that while the cushion relieves the irritated tissues, it also releases a controlled therapeutic dose of drug directly to the site of the infection, figure 3. If we can do this with a more biocompatible tissue conditioner, this would provide an even better solution. | 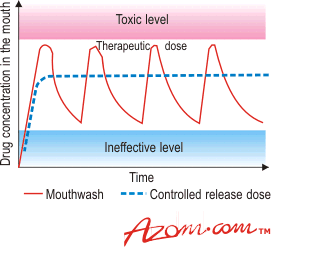
| | Figure 3. The principle of controlled release drugs. | Controlled Drug Release Systems Known widely as controlled release systems, drug-delivering devices found their way into medical applications during the 1970s with the development of a polypeptide-delivering polymer. Since then, a multitude of devices have been made, from basic systems that incorporate a drug into the matrix of a material, to ‘smarter’ polymers that deliver drugs when a certain enzyme or pH is encountered. The latter systems have the advantage of reacting to changes in their environment as they arise. Controlled release systems have been developed into such diverse devices as nicotine patches, contraceptive implants and ocular beads for the treatment of glaucoma. A Possible Solution The Materials To improve the biocompatibility of the tissue conditioners, ways of making gels without ethanol and subsequently with non-phthalate plasticisers were investigated. The first aim was achieved by moving to an 80/20 copolymer of n-butyl methacrylate and poly(ethyl methacrylate). Since this material is less dense than PEM alone, it is able to form a gel in a reasonable time without the need for ethanol to expedite the process. The most common plasticisers used in current tissue conditioners are dibutyl phthalate (molecular weight 278) and butyl phthalyl butyl glycolate (mwt. 336). As a substitute for these, I looked to high molecular weight citrates - the sense here being that, as well as the superior biocompatibility of citrates, in general at least, the larger the molecule, the slower it will leach. By running a variety of formulations through a rheometer, I found that ethanol-free tissue conditioners made with acetyltri-n-butyl citrate (mwt. 402) gelled in a similar time to the market-leading commercial conditioner. Incorporating the Drug The next step was to find out if the new tissue conditioner could release drug for long enough, and at a high enough concentration, to treat the candidal infection that often accompanies denture stomatitis. To investigate this, the broad antifungal drug, chlorhexidine, was incorporated into the tissue conditioner by ball milling the drug with the copolymer powder. As anticipated, adding the drug slowed the gelling process slightly, but this was solved by adding a concessionary 2% ethanol to the plasticiser. Drug was also added to other formulations of tissue conditioner for comparison. Testing Drug Release As a first approximation, drug delivery into 37°C distilled water was measured using UV/visual spectrophotometry. The new conditioner was found to release drug more slowly than the drug-loaded commercial material. However, what needed to be determined was whether the amount released was sufficient to kill C, albicans. Simple experiments showed that the drug-carrying conditioners (based on both the new conditioner and the commercial conditioner) killed the entire population of a C. albicans suspension (106 cells/ml) in less than an hour. This indicated that the methodology worked. It was also important to find out if these materials would still be effective after a period of use. This was determined by performing the same test after storing the samples in solution for a week. This time, the experimental conditioner achieved a 100% kill in four hours, while the commercial material took 46 hours. The commercial conditioner, it appeared, had leached so much of the drug in the first week, that its efficacy during the second week was compromised. Other Applications From the studies done to date, the new drug delivering tissue conditioner looks promising. With minor changes, the system could also be used in other circumstances. One possibility is to attach an amount of the drug-delivering gel to a tooth. This kind of system could benefit patients such as those undergoing radiotherapy for oral cancers, where a compromised immune system can result in oral infections becoming rife. A tooth-mounted gel could also be used to deliver fluoride. This would modify the surface structure of the teeth and make them more resistant to dental caries, a potential benefit in regions where natural fluoride levels in drinking water are low. Initial tests with fluoride-delivering gels have shown that a single loading of sodium fluoride can be delivered at a sustained rate over many weeks. Another application could be in maxillofacial surgery where tissue conditioner gels are already used as obturators to plug areas where tissue has been removed. A controlled release gel could conceivably deliver antibiotics to the site of surgery and possibly even release growth hormones to aid wound healing. As always, more work needs to be done. How much drug would a conditioner need to hold to clear up a real Candida infection? Will this amount be so low that ethanol can be removed from the system completely, or so high that the constituents won't form a gel? There are indubitably other questions which I’m sure will be pursued in the near future. If the system still looks useful once these points have been addressed, the next step will be a clinical trial. Summary It seems then that denture wearers are likely to have to wait a little longer before drug-delivering tissue conditioners reach the market. A little too late, of course, for George Washington, but in time for the Clintons and Blairs of this world, should the need arise. |