Alloy Types The alloys of titanium can be classified into three main groups as follows: • Alpha alloys- These are non-heat treatable and are generally very weldable. They have low to medium strength, good notch toughness, reasonably good ductility and have excellent properties at cryogenic temperatures. The more highly alpha or near alpha alloys offer high temperature creep strength and oxidation resistance. • Alpha-Beta alloys- These are heat treatable to varying extents and most are weldable with the risk of some loss of ductility in the weld area. Their strength levels are medium to high. Hot forming qualities are good but cold forming often presents difficulties. Creep strength is not usually as good as in most alpha alloys. • Beta alloys- Beta or near beta alloys are readily heat treatable, generally weldable, and offer high strength up to intermediate temperature levels. In the solution treated condition, cold formability is generally excellent. Crystallographic Forms The metallurgy of titanium is dominated by the crystallographic transformation which takes place in the pure metal at 882°C. Below this temperature, pure titanium has a hexagonal close packed structure known as alpha (α); above it, the structure is body centred cubic and termed beta (β). The fundamental effect of alloying additions to titanium is alteration of the transformation temperature and production of a two-phase field in which both alpha and beta phases are present. Elements having extensive solubility in the alpha-phase characteristically raise the transformation temperature and are called alpha stabilisers. Alpha Stabilisers Figure 1 typifies the binary phase diagram formed by addition of an alpha stabiliser (such as aluminium, oxygen, nitrogen or carbon) to titanium. Oxygen is added to pure titanium to produce a range of grades having increasing strength as the oxygen level is raised. Aluminium is the only other alpha stabiliser used commercially and is a major constituent of most commercial alloys. It is a very effective alpha-strengthening element at ambient and elevated temperatures up to about 550°C. The low density of aluminium is an additional advantageous feature but the amount that can be added is limited because of the formation of a brittle titanium-aluminium compound at aluminium contents exceeding about 8% by weight. | 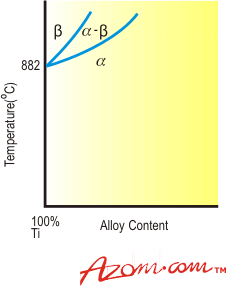
| | Figure 1. Effect of alpha-stabilising elements on titanium | The alpha phase is also strengthened by the addition of tin or zirconium. These metals have appreciable solubility in both alpha and beta phases and as their addition does not markedly influence the transformation temperature they are normally classified as neutral additions. As with aluminium, the beneficial ambient temperature hardening effect of tin and zirconium is retained at elevated temperatures. Figure 2 demonstrates schematically the phase diagram for titanium and a neutral element. | 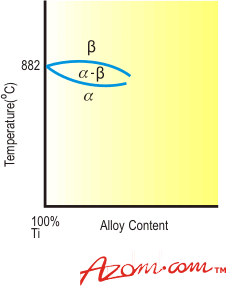
| | Figure 2. Effect of neutral elements on titanium. | Beta Stabilisers Elements that depress the transformation temperature, readily dissolve in and strengthen the beta phase and exhibit low alpha phase solubility are known as beta stabilisers. They can be divided into two categories according to their constitutional behaviour with titanium: • Beta-isomorphous elements • Beta-eutectoid elements. Beta-Isomorphous Elements Beta-isomorphous elements exhibit complete mutual solubility with beta titanium. Increasing addition of the solute element progressively depresses the transformation temperature to give the characteristic phase diagram shown in Figure 3. Molybdenum and vanadium are the most important beta isomorphous elements, while niobium and tantalum have also found application in some alloys. | 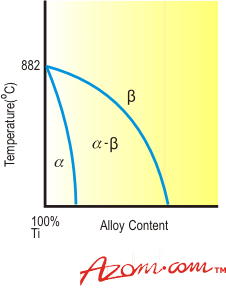
| | Figure 3. Effect of beta-isomorphous elements on titanium. | Beta-Eutectoid Elements Beta-eutectoid elements have restricted solubility in beta titanium and form intermetallic compounds by eutectoid decomposition of the beta phase. A representative phase diagram is illustrated in Figure 4. Elements of the beta-eutectoid type can be further subdivided into sluggish and active elements. Commercially important metals in the sluggish category are iron, chromium and manganese. Eutectoid decomposition of beta phase in the titanium-iron, titanium-chromium and titanium-manganese systems is so slow that intermetallic compound formation does not occur during normal commercial fabrication and heat treatment or during service and, therefore, for practical purposes the behaviour of iron, chromium and manganese can be likened to that of beta-isomorphous elements. | 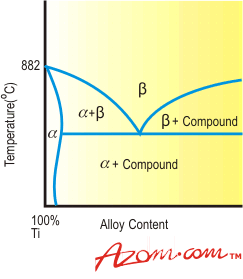
| | Figure 4. Effect of beta-eutectoid elements on titanium. | In contrast, copper and silicon form active eutectoid systems where below the eutectoid temperature the beta phase decomposes to alpha and intermetallic compounds within commercially acceptable times. As a result, controlled precipitation of the intermetallic compounds can be utilised to enhance the strength of titanium alloys containing appropriate concentrations of silicon or copper. Advantages of Beta Alloys In addition to strengthening the beta phase, beta stabilisers have two other important advantages as alloying constituents. Beta titanium has an inherently lower resistance to deformation than the alpha modification and therefore elements which increase and stabilise the beta phase tend to improve alloy fabricability during both hot and cold working operations. Addition of sufficient beta stabiliser to titanium compositions also confers a heat treatment capability which permits significant strengthening to be achieved by controlled decomposition of beta phase to alpha phase during the heat treatment process. Summary Discussion up to this point has been concerned with the constitutional behaviour of commercially significant alloying elements, all of which form substantial solid solutions with titanium. This means that solute atoms replace or substitute for titanium atoms in the two crystallographic forms of titanium. In contrast, the elements oxygen, nitrogen, carbon and hydrogen, which are always present in titanium, form interstitial solid solutions in which the solute atoms are located in the “holes” or interstices between the titanium atoms. Oxygen, nitrogen and carbon are alpha stabilisers. Hydrogen, on the other hand, dissolves preferentially in the beta phase, has negligible solubility in alpha and is thus classified as a beta stabiliser. Hydrogen is further distinguished from the other interstitial elements by the fact that its diffusion rate in titanium is rapid at elevated temperatures. |