Nov 12 2014
Researchers from the Oak Ridge National Laboratory (ORNL) of the Department of Energy have made a breakthrough in comprehending vanadium oxide, a typical transition metal oxide, by calculating the thermodynamic forces that cause the transformation.
The results have been recorded in Nature, November 10, online issue. John Budai co-led the study with Jiawang Hong, a colleague in the Materials Science and Technology Division of ORNL.
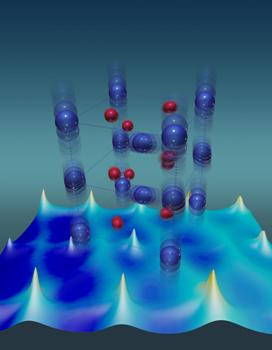 Vanadium atoms (blue) have unusually large thermal vibrations that stabilize the metallic state of a vanadium dioxide crystal. Red depicts oxygen atoms. Credit: ORNL
Vanadium atoms (blue) have unusually large thermal vibrations that stabilize the metallic state of a vanadium dioxide crystal. Red depicts oxygen atoms. Credit: ORNL
Vanadium oxide enhances storage media and recording, improves strength of structural alloys and provides color to synthetic jewels. In the future it may be used as optical shutters turning opaque on satellites to hinder obtrusive signals, nanoscale actuators for switches and field-effect transistors for electronics manipulation in spintronics and semiconductors in devices maneuvering magnetic spin.
They may also be used in energy-efficient "smart windows" coated with vanadium dioxide interspersed with impurities for controlling light and heat transmission.
Before these experiments were conducted by the ORNL team, the total heat amount absorbed during the transition of vanadium dioxide from insulator to metal was known. However, the amount of entropy due to electrons and the amount due to atomic vibrations was unknown.
The present achievement of the team was accomplished by combining neutron scattering and X-ray tools making lattice dynamics calculations possible. Also the team used a calculation method developed by Linköping University, Sweden’s Olle Hellman for determining anharmonicity, which is a value of non-linearity in bond forces present between atoms. It is important that the computations done by Hong concur with experiments as they can be used for new forecasts for other materials.
ORNL researchers determined incoherent neutron scattering at ORNL's Spallation Neutron Source (SNS) to ascertain the phonon spectra at different temperatures and to determine coherent inelastic and diffuse X-ray scattering at the advanced photon source (APS) of Argonne National Laboratory's to study collective vibrations in pristine crystals.
The SNS’s large neutron flux enabled neutron measurements and X-ray measurements were possible with the high-resolution obtained with high APS brightness.
According to the findings, it was found that in the metal state, the vanadium dioxide lattice is anharmonic. When heated to a temperature of 340K, vanadium oxide undergoes transition from insulator to metal. Hong made significant contributions in terms of quantum molecular dynamics calculations.
"The theory not only provides us deep understanding of the experimental observations and reveals fundamental principles behind them, but also gives us predictive modeling, which will accelerate fundamental and technological innovation by giving efficient strategies to design new materials with remarkable properties."
Jiawang Hong
Many materials other than vanadium dioxide show metal-to-insulator transition; however, how exactly lattice vibrations control phase stability is still a mystery. This basic research will help in steering the advancement of energy-efficient materials.