Jun 16 2015
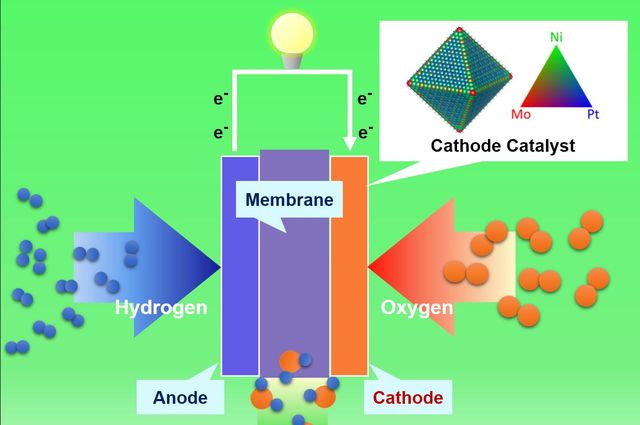 Diagram of a proton exchange membrane fuel cell created using a surface engineering technique called “surface doping.” Credit: Yu Huang Lab/UCLA
Diagram of a proton exchange membrane fuel cell created using a surface engineering technique called “surface doping.” Credit: Yu Huang Lab/UCLA
Researchers at the UCLA Henry Samueli School of Engineering and Applied Science have led a team that has used a three-metal compound to create nanostructures, which can produce fuel cells with improved durability and efficiency at a lower cost.
Many problems have prevented widespread adoption of fuel cells, and this development could help increase its adoption. Proton exchange membrane fuel cells are considered to be a promising technology for clean energy applications.
In these cells, reaction takes place between oxygen from air and hydrogen fuel, and electricity is produced. In conventional car engines greenhouse gases and pollutants are emitted, however, in this process water is created as exhaust. Hence, proton exchange membrane fuel cells could be used for zero-emission vehicles.
In proton exchange membrane fuel cells, metals catalyze the chemical processes. For the oxygen reduction reaction, platinum is typically used as the catalyst. Platinum is expensive, and has hence prevented widespread adoption of fuel cells. Alternative catalysts such as platinum–nickel compounds have been considered, but they have not been viable due to their insufficient durability.
The researchers had to create a more durable, more efficient, and cost-effective fuel cell. They adopted the “surface doping” surface engineering technique to develop a fuel cell with the desired properties. Molybdenum was added as a third metal to the platinum-nickel nanostructure surface. This increased the stability of the alloy surface, and also prevented loss of platinum and nickel.
Comparison between commercial platinum-carbon compound catalysts and platinum-nickel-molybdenum surface nanostructure catalysts found that the latter demonstrated 81 times better efficiency. The platinum-nickel catalysts demonstrated 66% efficiency rate, while the platinum-nickel-molybdenum compound retained approximately 95% of its efficiency.
We showed that the addition of a third transition metal enables improvement in both efficiency and durability to bring down long-term costs. In addition, the surface doping approach may also apply to a broad range of catalysts and opens up a new route for catalyst engineering for the search of high performance catalysts for environment protection, energy generation and chemical productions.
Yu Huang, UCLA Associate Professor of Materials Science & Engineering
This study has been published in the journal Science.