May 18 2017
Normally, oxide (dolomite: MgO, CaO) a raw material for magnesium metal does not absorb microwave energy properly and does not produce heat.
However in this research when electrically conductivity ferrosilicon (FeSi) used as a reducing agent was blended with the raw dolomite material and formed into an antenna* structure, it became easier to absorb the microwave energy and decrease the temperature.
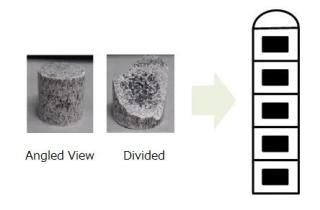 (Left) Actual pellet of dolomite and ferrosilicon. White portion is dolomite rich, and the black portion is ferrosilicon rich. Ferrosilicon is concentrated in the center portion. (Right) Five stacked to form the microwave wavelength (antenna structure). (CREDIT: Tokyo Institute of Technology)
(Left) Actual pellet of dolomite and ferrosilicon. White portion is dolomite rich, and the black portion is ferrosilicon rich. Ferrosilicon is concentrated in the center portion. (Right) Five stacked to form the microwave wavelength (antenna structure). (CREDIT: Tokyo Institute of Technology)
Contact point heating and internal heating, which are microwave features, were observed, and the average reaction temperature for this smelting was dropped from the conventional 1,200 - 1,400 °C to 1,000 °C.
The research results have been published in the April 12th issue of Scientific Reports, the online edition of the sister magazine of the UK scientific journal Nature.
Research Highlights
- Formulated a new smelting technique for magnesium, which is the lightest industrial-used metal
- Devised a forming technique for materials to improve thermal conductivity during microwave irradiation
- Energy savings anticipated for smelting other useful metals
Background
Presently, the smelting of magnesium metal is mostly performed using the Pidgeon technique (thermal reduction technique) where the material temperature is elevated using a large quantity of coal as the heat source. Approximately 80% of magnesium metal is produced in China. A large quantity of coal is consumed for smelting, causing the generation of the air pollutant PM 2.5 (fine particulate matter) and the emission of carbon dioxide into the atmosphere, which are big problems.
The Pidgeon technique is used for heating dolomite ore and silicon iron to high temperatures and then cooling the evaporated magnesium to attain magnesium metal.
2MgO (s) + 2CaO (s) + (Fe)Si (s) ? 2Mg (g) + Ca2SiO4 (s)+ Fe (s)
* s: Solid, g: Gas
Dolomite mineral: MgO, CaO; Ferrosilicon: FeSi
Heat source: Coal
Research Achievements
Typically, dolomite is a poor absorber of microwave energy and does not produce heat. However, by employing ferrosilicon as the reducing agent, devising the shape of the raw material pellet obtained by mixing dolomite and ferrosilicon and making it as an antenna so that it has a resonance structure of 2.45 GHz (same as the frequency for microwave ovens), it was possible to confine the microwave energy to the pellet.
About 1 g of magnesium metal was smelted effectively in a small-scale experimental reactor. Also, so as to accurately estimate the energy, a demonstration furnace around five times larger than the experimental furnace was built and experiments were conducted, resulting in the effective smelting of approximately 7 g of magnesium metal. This can decrease energy by 68.6% compared with the conventional technique.
Future Developments
This achievement of saving energy while smelting magnesium metal has led to the possibility of this method being developed and used in high temperature reduction process of oxides. In the future, by additionally developing this research, it can be applied to the smelting of other metal materials to save energy with metals, steel, chemistry and materials, which have not progressed, and help lessen carbon dioxide, which is one of the major causes of global warming.