Jun 23 2017
Chemists from the University of Kentucky and the Institute of Physics Research of Mar del Plata in Argentina recently reported a new way for triggering a basic step in the mechanism of photosynthesis, providing a process that proves to be promising in the development of a new technology that will help reduce the levels of carbon dioxide.
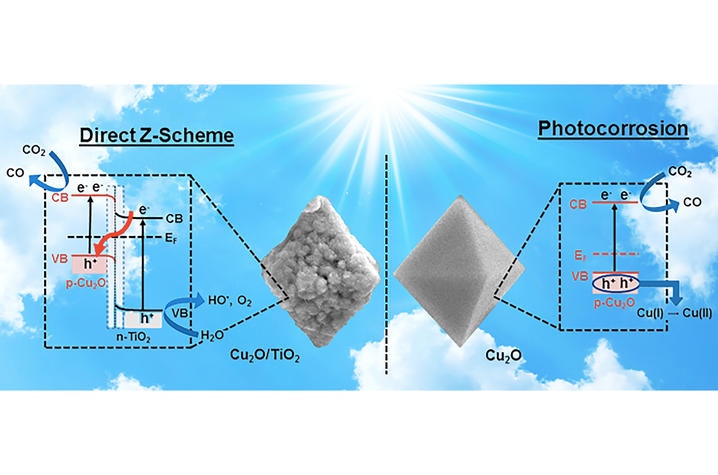 Cu2O (right) that gets photocorrosion compared to Cu2O/TiO2 (left) that operates under a Z-scheme to reduce CO2. Artwork by Ruixin Zhou, UK doctoral student of chemistry.
Cu2O (right) that gets photocorrosion compared to Cu2O/TiO2 (left) that operates under a Z-scheme to reduce CO2. Artwork by Ruixin Zhou, UK doctoral student of chemistry.
The research team is headed by Marcelo Guzman, an Associate Professor of Chemistry in the UK College of Arts and Sciences, and Ruixin Zhou, a Doctoral Student working with Guzman. They used a synthetic nanomaterial that incorporates the greatly reducing power of cuprous oxide (Cu2O) with a coating of oxidizing titanium dioxide (TiO2) in order to prevent the loss of copper (I) ion in the catalyst. The catalyst produced from Cu2O/TiO2 is capable of transferring electrons for bringing about reductions in the atmospheric greenhouse gas carbon dioxide (CO2) while simultaneously breaking the molecule of water (H2O). The novel feature of this catalyst for electron transfer imitates the “Z-scheme” mechanism from photosynthesis.
In a report published in Applied Catalysis B: Environmental, the Researchers explain that electrons get transferred to CO2 if the catalyst is exposed to sunlight. This transfer takes place in a process that resembles the manner in which photosystems 1 and 2 function in nature.
Developing the materials that can be combined to reduce CO2 through a direct Z-scheme mechanism with sunlight is an important problem. However, it is even more difficult to demonstrate the process actually works. From this scientific viewpoint, the research is contributing to advance future technology for carbon sequestration.
Ruixin Zhou, Doctoral Student
Several Scientists have been pursuing this task for a prolonged time period but the challenge here is to establish the fact that both components of the catalyst interact in order to help the electronic properties of a Z-scheme mechanism. Despite the possibility of using a wide range of materials, the main aspect of this research refers to the fact that the catalyst is not made of scarce and extremely costly elements such as iridium and rhenium to drive the reactions with sunlight energy approaching the Earth’s surface. Corrosion resistant TiO2 was used by the catalyst in order to apply a white protective coating to octahedral particles of red Cu2O.
The Researchers designed a number of experiments to test the hypothesis that the catalyst works through a Z-scheme instead of employing a double-charge transfer mechanism. The proposed Z-scheme was verified to be operational by the measured carbon monoxide (CO) production from CO2 reduction, the characterized optical and electronic properties of the individual components and catalyst, and the identification of hydroxyl radical (HO•) intermediate from H2O oxidation en route to form oxygen (O2).
The research next focuses on improving the approach by analyzing a variety of catalysts and then identifying the one that is extremely efficient and capable of transforming CO2 into chemical fuels such as methane. This will help in developing a new technology to supply affordable and clean alternative energy sources and also to deal with the problem of constant consumption of fossil fuels and increasing levels of greenhouse gases.
This research was partially supported by the U.S. National Science Foundation, UK and by two Argentinean agencies (ANPCyT and CONICET).