Over the past decade the symbiotic nature of Quality by Design (QbD) and Process Analytical Technology (PAT) has become clear. In the simplest of terms QbD systematically identifies what must be measured, and controlled, while PAT provides the necessary measurements, in the required timeframe. Wet granulation, and more specifically high shear wet granulation (HSWG), is a valuable process for the pharmaceutical industry, particularly for the preparation of tableting blends, but it remains challenging from this perspective. There is still debate over what can be most usefully measured and consequently the most appropriate PAT.
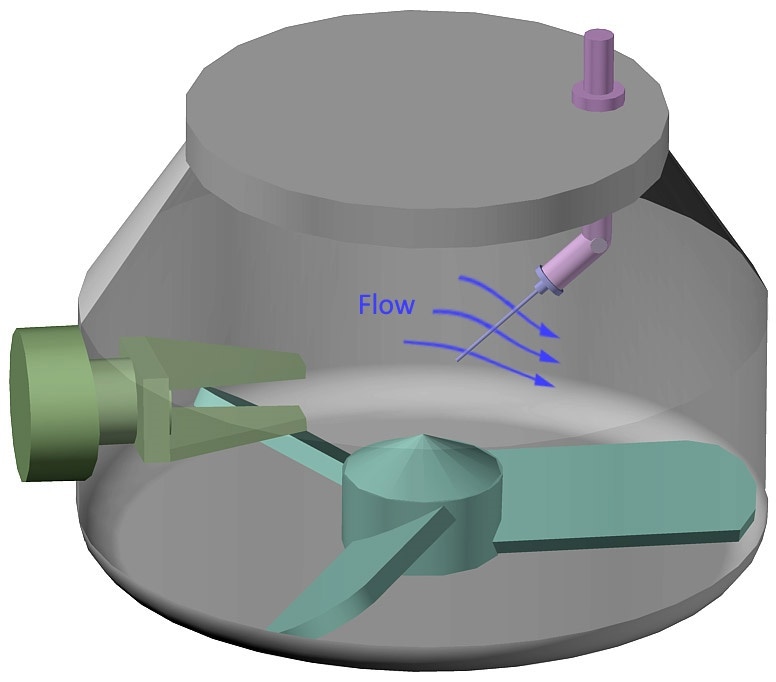
Our latest whitepaper, featured in Pharmaceutical Technology, demonstrates the value of dynamic powder flow measurements for the development and optimisation of wet granulation processes, drawing on data from two experimental studies. It shows how:
- the FT4 Powder Rheometer® can be used to measure granule properties that correlate directly with the Critical Quality Attributes (CQAs) of tablets.
- the Lenterra Flow Sensor system, a new PAT for real-time measurement, enables complementary, continuous in-line monitoring.
Used together these technologies present an integrated solution for HSWG monitoring and control.
To access your copy please visit the Pharmaceutical Technology website.