Nov 16 2018
In the near future, implantable medical devices like sensors and pacemakers could be energized by a glucose-powered biofuel cell in which the electrodes are made from cotton fiber. The new fuel cell, which offers double the power of traditional biofuel cells, could be combined with supercapacitors or batteries to offer a hybrid power source for the medical devices.
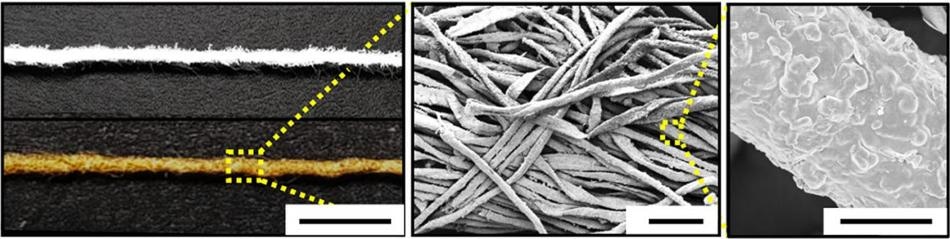 Image shows plain cotton fibers and metallic cotton fibers used as electrodes in a new biofuel cell. (Image credit: Georgia Tech/Korea University)
Image shows plain cotton fibers and metallic cotton fibers used as electrodes in a new biofuel cell. (Image credit: Georgia Tech/Korea University)
Scientists from the Georgia Institute of Technology and Korea University used gold nanoparticles arranged on the cotton to develop high-conductivity electrodes that helped enhance the efficiency of the fuel cell. In this way, they were able to tackle one of the major issues that restrict the performance of biofuel cells—linking the enzyme used to oxidize glucose with an electrode.
The gold electrodes are manufactured using a layer-by-layer assembly method—offering the electrocatalytic cathode as well as the conductive substrate for the anode—which helped improve the power capacity to approximately 3.7 mW/cm2. The outcomes of the study were published in the Nature Communications journal on October 26th, 2018.
We could use this device as a continuous power source for converting chemical energy from glucose in the body to electrical energy. The layer-by-layer deposition technique precisely controls deposition of both the gold nanoparticle and enzyme, dramatically increasing the power density of this fuel cell.
Seung Woo Lee, Assistant Professor, Woodruff School of Mechanical Engineering, Georgia Tech.
Fabrication of the electrodes starts with porous cotton fiber made up of multiple hydrophilic microfibrils, which are cellulose fibers having hydroxyl groups. Gold nanoparticles with a diameter of around 8 nm are then deposited onto the fibers using organic linker materials.
In order to develop the anode for oxidation of the glucose, the scientists applied glucose oxidase enzyme and an amine-functionalized small molecule called TREN in alternating layers. The cathode, at which the oxygen reduction reaction occurs, was made of the gold-covered electrodes, which possess electrocatalytic capabilities.
“We precisely control the loading of the enzyme,” Lee said. “We produce a very thin layer so that the charge transport between the conductive substrate and the enzyme is improved. We have made a very close connection between the materials so the transport of electrons is easier.”
When compared to a nylon fiber, the porosity of the cotton enabled an increase in the number of gold layers. “Cotton has many pores that can support activity in electrochemical devices,” explained Yongmin Ko, a visiting faculty member and one of the co-authors of the paper. “The cotton fiber is hydrophilic, meaning the electrolyte easily wets the surface.”
In addition to enhancing the conductivity of the electrodes, the cotton fiber could optimize the biocompatibility of the device, as it is designed to function at low temperatures to enable use inside the body.
Implantable biofuel cells undergo degradation in due course, whereas the new cell developed by the U.S. and Korean team provides increased long-term stability. “We have a record high power performance, and the lifetime should be improved for biomedical applications such as pacemakers,” Lee said.
Currently, implantable devices such as pacemakers are powered by batteries that last for years; however, these devices may still demand battery replacement in a procedure that needs surgery. Lee further stated that the biofuel cell could supply an uninterrupted charge for those batteries, potentially prolonging the time that devices may operate without battery replacement.
Furthermore, the biofuel cell could be employed to power devices that are temporarily used. Such devices might be implanted to enable timed release of a drug; however, these devices would biodegrade eventually with no need for surgical removal. For such applications, battery would not be included, and the biofuel cell could supply the less power needed.
Future objectives of the study include the development of a functional implantable power source, and representing operation of the biofuel cell with an energy storage device.
We want to develop other biological applications for this. We’d like to go farther with other applications including batteries and high-performance storage.
Seung Woo Lee, Assistant Professor Woodruff School of Mechanical Engineering, Georgia Tech.
Additional members of the research team included Cheong Hoon Kwon, Dongyeeb Shin, Minseong Kwon, and Jinhan Cho of Korea University; Jinho Park of Georgia Tech; and Wan Ki Bae of SKKU Advanced Institute of Nano Technology at Sungkyunkwan University.
This study was supported by a National Research Foundation (NRF) grant funded by the Korean Ministry of Science, ICT & Future Planning (MSIP) (2018R1A2A1A05019452; 2016M3A7B4910619) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF2017R1A6A3A04003192).