Mar 20 2019
Platinum atoms, weakly held by ligands, have high activity and resist combining into nanoparticles.
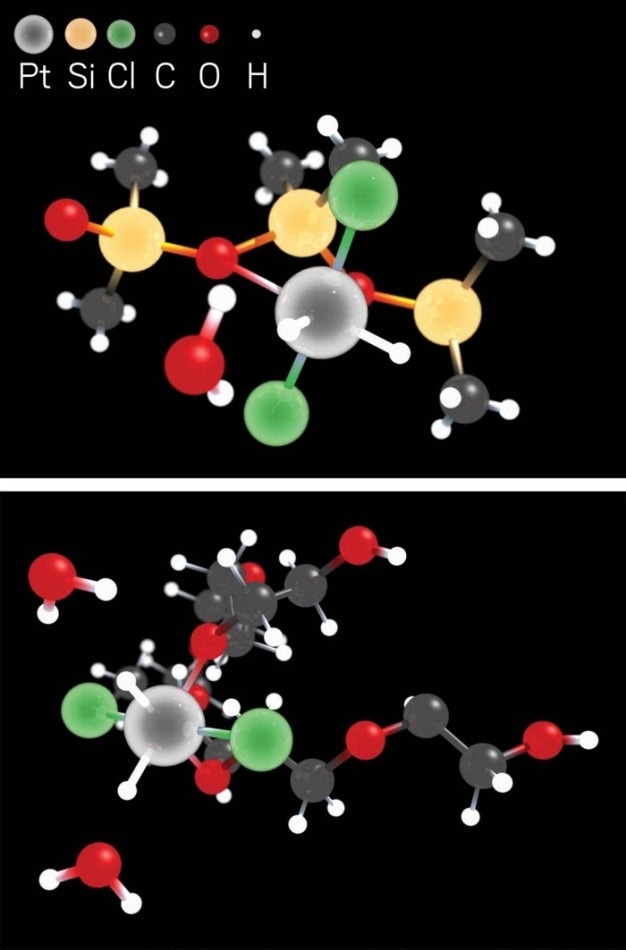 A platinum-based liquid-phase catalyst remains stable due to the metal’s interactions with hydrochloride species, PDMS ligands (top), and PEG ligands (bottom). (Image credit: Kairui Liu/DICP)
A platinum-based liquid-phase catalyst remains stable due to the metal’s interactions with hydrochloride species, PDMS ligands (top), and PEG ligands (bottom). (Image credit: Kairui Liu/DICP)
When researchers gently restricted individual metal atoms in a surfactant solution, they ultimately created a catalyst that is chemically selective, highly active, and integrates the benefits of traditional liquid-phase and solid-state catalysts.
In order to increase the application of expensive noble metals, including platinum, the team has now developed several techniques for separating each catalytic atom, usually on solids. The molecular uniformity of single-atom catalysts (SACs), apart from utilizing the resources efficiently, eases the task of inferring reaction mechanisms, which are crucial for enhancing the performance of catalysts. While such uniformity is a trademark of liquid-phase molecular catalysts, it is uncharacteristic of solid catalysts, which do not contain isolated metal atoms. However, only a few techniques are available for making SACs, and metal atoms present in liquid catalysts do not invariably stay put, and at times they migrate, creating comparatively inactive clumps.
In order to make this novel, clump-resistant liquid catalyst, Z. Conrad Zhang of Dalian Institute of Chemical Physics, Shi Bai of the University of Delaware, and coworkers performed a study in which a platinum salt was allowed to react with alcohols in a poly(dimethylsiloxane)-poly(ethylene glycol) (PDMS-PEG) solution. The reaction eventually resulted in a catalytic liquid containing isolated platinum atoms that are resistant to aggregation because of their interactions with oxygen atoms and hydrochloride species in the PEG and PDMS ligands. When the catalyst was tested in hydrosilylation reactions—commonly utilized by the silicone sector to create carbon-silicon bonds—it was observed that the novel material was reusable, stable, and as much as 100 times as active as regular platinum catalysts.
According to Daniel E. Resasco, catalysis specialist from the University of Oklahoma, the significance of the study is that the researchers selected ligands that adhere to the metal atoms weakly enough to enable high catalytic activity, but robustly enough to inhibit them from forming nanoparticles.
Resasco further observed that this feeble interaction does not considerably modify the electronic state of the metal, leaving the active sites “pristine,” which are suitable for scientists performing fundamental analyses to understand the mechanism of reactions.
According to Bert M. Weckhuysen of Utrecht University, an expert in studying catalysts, since the catalyst is a liquid, a number of characterization methods and spectroscopy techniques can be used by chemists to examine it and thus expose the mechanistic insights.