Mar 29 2019
Dual-ion batteries (DIBs), in which cations and anions take part in the electrochemical redox reaction, are one of the most potential candidates that fulfill the demands of the cost-effectiveness of commercial applications.
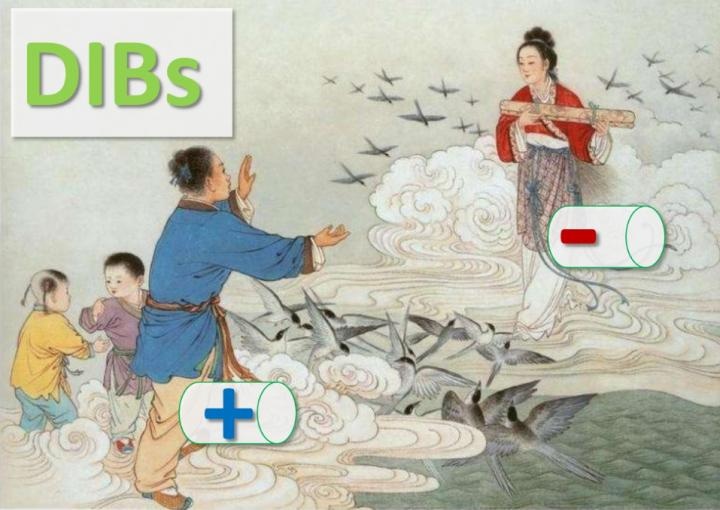 Anions and cations in DIBs act like the Cowherd and the Weaver Girl. (Image credit: Tang Yongbing)
Anions and cations in DIBs act like the Cowherd and the Weaver Girl. (Image credit: Tang Yongbing)
They have many advantages such as excellent safety, high working voltage, and eco-friendliness than the usual lithium-ion batteries (LIBs).
Researchers, under the guidance of Prof. Tang Yongbing and Dr Zhou Xiaolong, at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, in collaboration with other researchers published an invited review article titled “Beyond Conventional Batteries: Strategies towards Low-Cost Dual-Ion Batteries with High Performance” in Angewandte Chemie International Edition.
DIBs are of great interest across the world due to their ease of recycling, low cost, low environmental impact, high working voltage, and so on. But the conventional double-carbon structure of DIBs possesses a low energy density owing to the limitation of theoretical capacity and compaction density of graphite.
In 2016, Prof. Tang’s team developed a new aluminum–graphite DIB that was capable of combining the electrodes. It produced a new, cheap, highly efficient aluminum–graphite DIB system using aluminum foil, which is less expensive and eco-friendly, as not only the cathode active material but also the current collector and graphite as the anode material.
The anion and cathode are similar to the two lovers in a Chinese fairy tale, Cowherd and the Weaver Girl, who could meet only once in a year on a magpie bridge in the sky. The two lovers are parted by the vast Milky Way Galaxy (electrolytes); however, using magpie bridge (ion channel), they contact each other (discharge), and later go back to their original places (charge). This sequence goes on repeatedly.
The major differences between LIBs and DIBs are outlined as follows: When charging, intercalation of anions into cathodes occurs, leading to a different electrochemical energy storage mechanism and a high working voltage. The electrolytes are regarded as active materials in DIBs because the anions originate from the electrolytes. Hence, in the charge–discharge process, cations and anions are separated and brought together in the electrolyte.
The researchers have also extended the discovery of integrated design to the alkali-rich (alkaline earth) metal-ion battery system. The effective development of a cheap and eco-friendly sodium-based DIB—the potassium-ion-based DIB—and the high-working-voltage calcium-ion battery at ambient temperature by the researchers laid a firm groundwork for the industrial application of such integrated technology.