May 29 2019
A team of scientists headed by Prof. XU Jie from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences has created a surface acidity- and selectivity-tunable manganese oxide catalyst via a surface modification method.
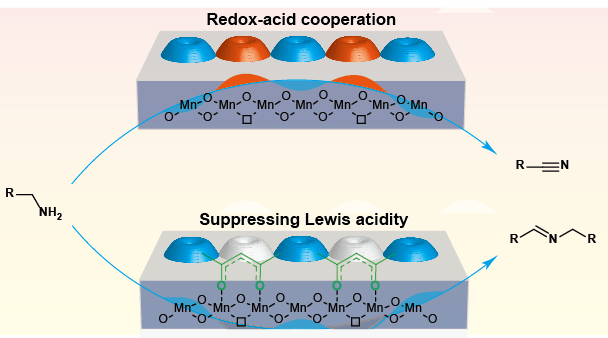 Selectivity-tunable amines oxidation over acetylacetones-modified manganese oxide catalyst. (Image credit: JIA Xiuquan)
Selectivity-tunable amines oxidation over acetylacetones-modified manganese oxide catalyst. (Image credit: JIA Xiuquan)
The study outcomes have been reported in Nature Communications.
Surface properties of transition metal oxides play a key role in their catalytic applications. Although several reports are analyzing the surface chemisorption of organic molecules on metal oxides, it is not obvious how adsorption of organic modifiers can be leveraged to optimize the catalytic properties of metal oxides.
The scientists used enolic acetylacetones to alter the surface Lewis acid properties of manganese oxide catalysts. This allowed rational control of the oxidation selectivities of structurally varied arylmethyl amines so they could change from nitriles to imines.
The stable variation of acetylacetones deeply affected the redox-acid cooperative catalysis of MnOx by suppressing the surface Lewis acidity of the catalysts. In the aerobic oxidation reaction of benzylamine, using unmodified MnOx as catalyst, nitrile was acquired with a yield of 86.5%. On the contrary, the MnOx altered by acetylacetones synthesized imine with a yield of 90.6% under identical conditions.
The present research illustrates an example of a selectivity-switchable metal oxide catalyst with an organic switch to adjust its surface properties. This may offer future insights into the surface structure-activity links of metal oxide catalysts.