Jun 24 2019
According to new research from a collaborative team based in Japan, the complete story of chemical reactions in flowing fluids is not revealed by the before and after comparisons, such as those in a chemical reactor.
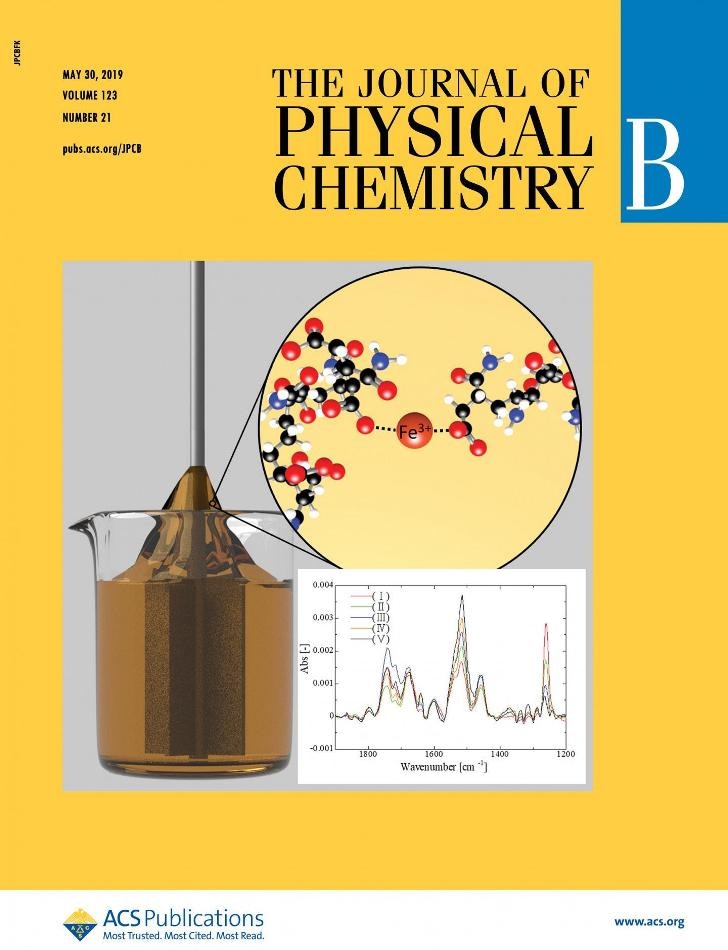 A temporal reaction occurred during the flowing of fluids indicated a fundamental structural change in the fluids. (Image credit: FIGURE ADAPTED with permission from the cover of J. Phys. Chem. B 2019, 123, 21, 4587-4593. Copyright © 2019 American Chemical Society)
A temporal reaction occurred during the flowing of fluids indicated a fundamental structural change in the fluids. (Image credit: FIGURE ADAPTED with permission from the cover of J. Phys. Chem. B 2019, 123, 21, 4587-4593. Copyright © 2019 American Chemical Society)
The scientists published their paper on May 6th, 2019 in the Journal of Physical Chemistry B, a journal of the American Chemical Society. The outcomes were reported on the journal’s cover.
The research group studied the change in the solution of dissolved polymers upon addition of Fe3+ solution. These kinds of solutions are used to better control variables in numerous fields, such as manufacturing. For example, in automobile manufacturing, the solutions help attain a complete uniformity of paint coverage and control over how much a material shrinks or expands under different temperatures.
Typically, scientists analyze a solution before a reactant—for example, Fe3+ solution—is added, and again after the reaction occurs.
In other words, if a fluid property such as the viscosity of the solution is higher after the reaction than before, we would expect that an increase in viscosity occurs from the reaction during flow.
Yuichiro Nagatsu, Associate Professor, Department of Chemical Engineering, Tokyo University of Agriculture and Technology
Nagatsu is also the corresponding author of the paper.
Nagatsu and the group found out that the before and after comparison is not as dependable as assumed earlier. They noticed a rise in viscosity in the solution during a chemical reaction to Fe3+; however, the solution had thinned back out by the end of the reaction.
They validated their chemical observations with infrared spectroscopy, which enables scientists to look at microscopic interactions without vast preparation that could further disturb the sample.
Flow dynamics contribute to microscopic variations within these chemical reactions—molecules stripping other molecules of electrons and the like—that basically alter the composition of the solution. However, viscosity is referred to as a macroscopic variation—it illustrates the solution as a whole rather than the individual interactions on the microscopic level.
It is extremely strange for such a solution to change through such macroscopic phases just to lose the characteristics at the end of a chemical reaction, reports Nagatsu. This insight could have significant implications across environmental, biological, and industrial fields.
“Our ultimate goal is to establish a new research area to understand chemically reacting flow involving the diagnosis of molecule structure,” Nagatsu stated. He also remarked about the strategies to create a new method to control fluid dynamics using their new understanding of interactions.
This research was supported by a grant-in-aid for scientific research from the Japan Society for the Promotion of Society (JSPS KAKENHI Grant No. 22686020).